Compositions and methods for tissue repair
a tissue and composition technology, applied in the field of tissue compositions and methods, can solve the problems of organ or tissue failure, frequent, expensive, serious problem in health care, and limited organ transplantation, and achieve the effect of facilitating the repair of the injured tissu
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Reagents
[0119]Immulon 1B, untreated 96-well plates (CoStar), and untreated 35 mm dishes (CoStar) were purchased from Fisher Scientific (Pittsburg, Pa.). Human umbilical vein endothelial cells (HUVECs), cell culture media and supplements were purchased from Lonza (Basel, Switzerland). EMD peptides as shown in SEQ ID Nos: 1-4, were synthesized by Commonwealth Biotechnologies Inc., (Richmond, Va.). Amino acid analysis was performed on the peptides to verify the amino acid sequence.
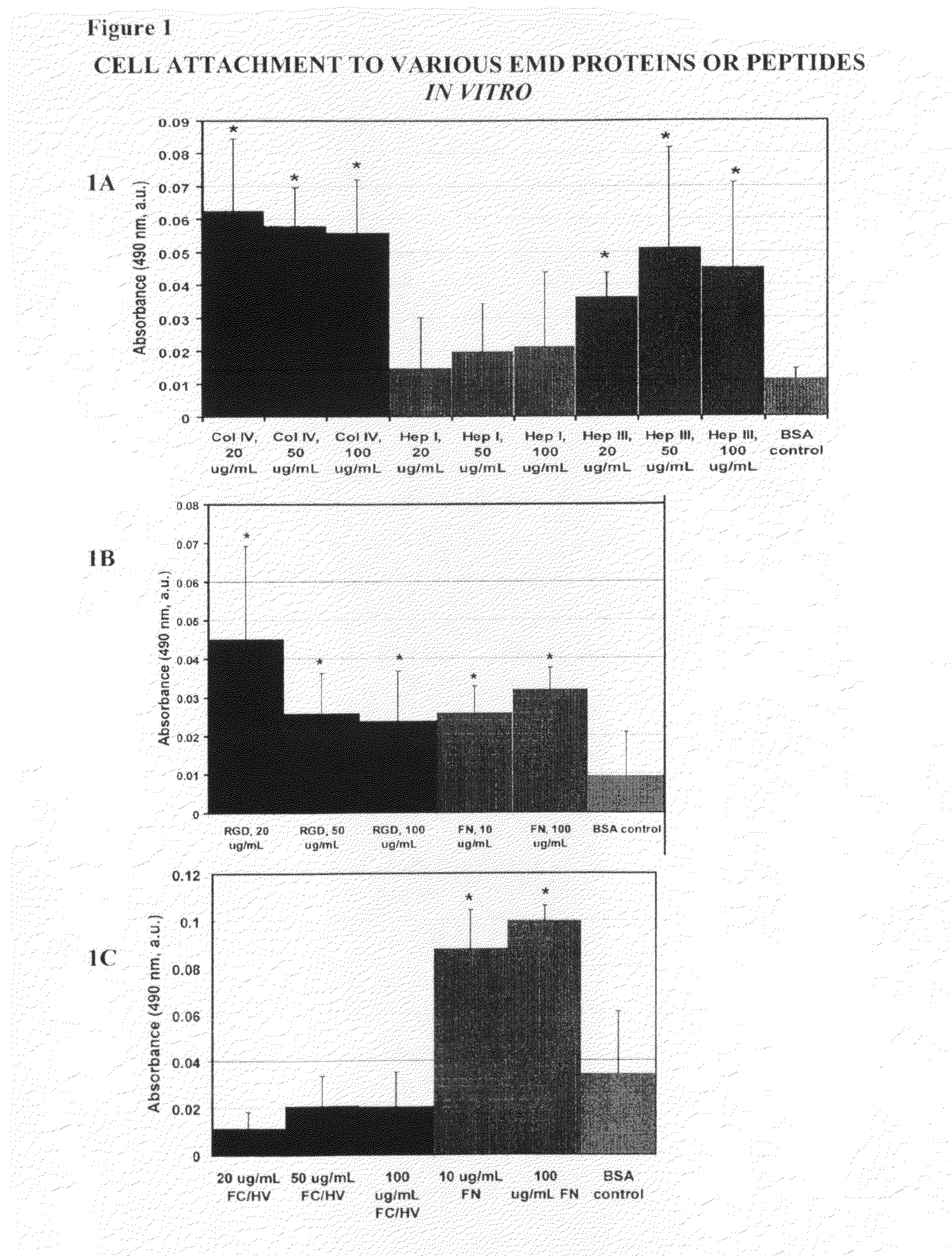
[0120]For the adhesion studies 96-well Immulon 1B plates were used. Various concentrations of the EMD peptides (SEQ ID Nos: 1-4) were tested for their ability to bind cells, and compared to the full-length protein, from which the EMD peptides were derived as positive controls. Bovine Serum Albumin (BSA) treated wells were used as a negative control. Full-length collagen IV, and the EMD peptides Hep I, Hep III, FCHV, and RGD were tested at concentrations of 20 μg / ml, 5...
example 2
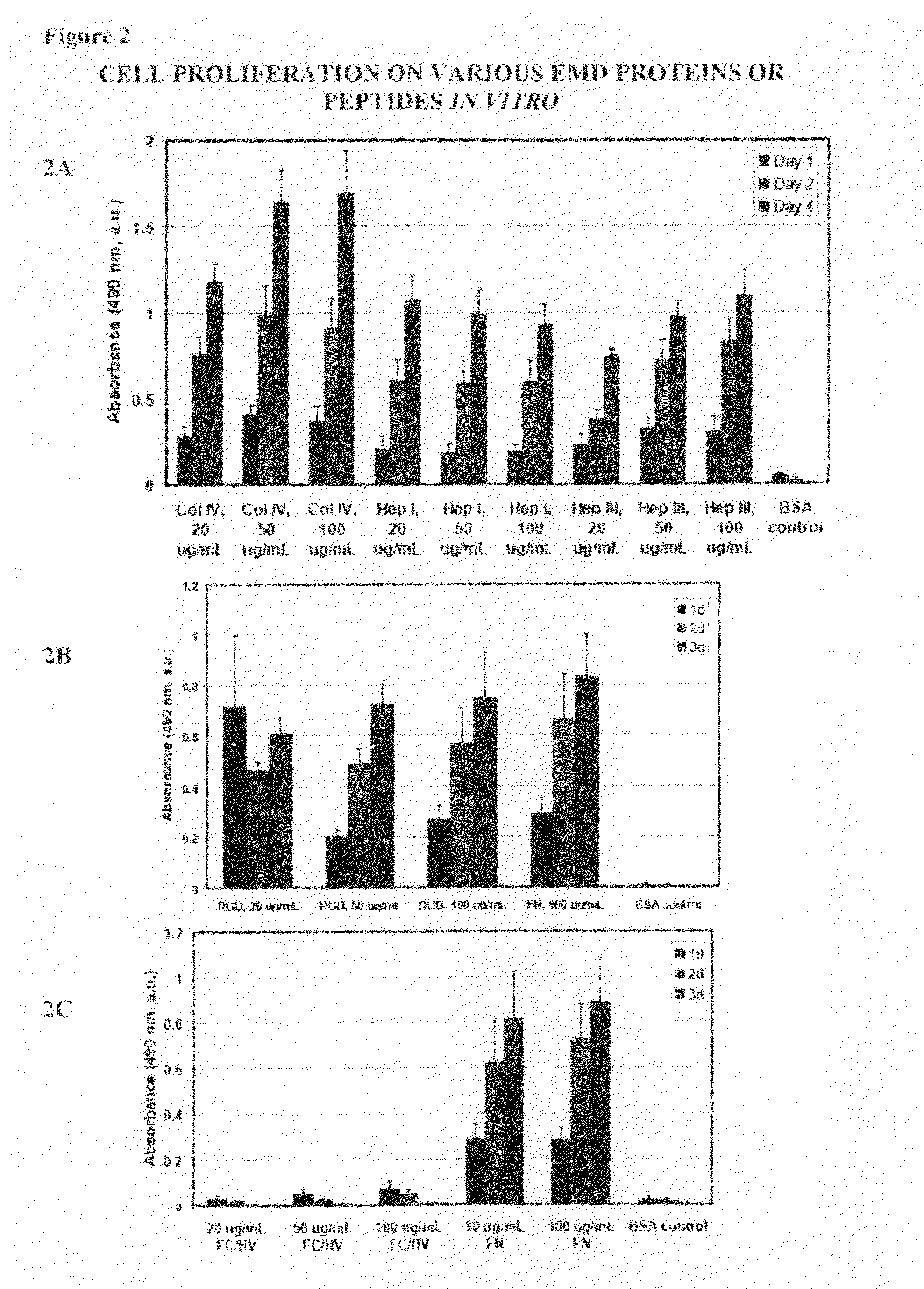
[0122]To determine if EMD peptides can activate cells by stimulating cellular proliferation, 96-well plates coated using the same protocol as described above for the cell attachment assay was used. Following treatment of the wells, HUVEC cells (Lonza, Basel Switzerland) were then added to the wells at a concentration of 5×103 to 1×104 cells per well and incubated under standard culture conditions for 1, 2, 3 or 4 days. Cell proliferation was then determined at the end of the incubation period using an MTS tetrazolium / formazan assay (Promega, Madison, Wis.) according to the manufacturer's instructions.
[0123]As shown in FIG. 2, full length proteins Col IV and fibronectin and EMD peptides Hep I, (SEQ ID NO:1) Hep III (SEQ ID NO:2), and RGD (SEQ ID NO:4) all showed increased cellular proliferation compared to cells cultured in wells coated with BSA. Notably, cells cultured in wells coated with FCHV (SEQ ID NO:3) was not different from that of cells cultured in we...
example 3
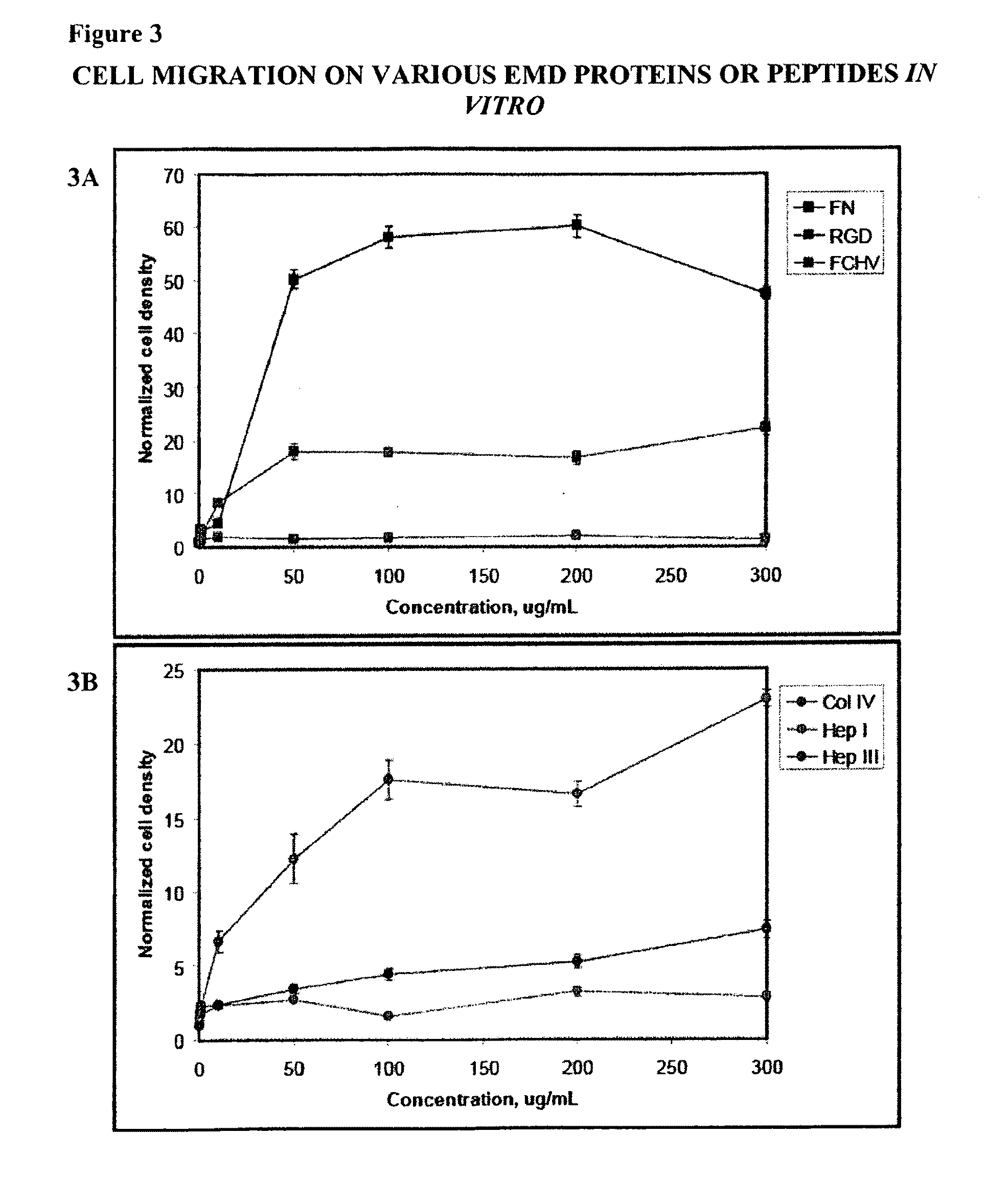
[0124]In order to test the ability of EMD peptides to recruit cells haptotactic migration assays with Hep I (SEQ ID NO:1), Hep ITT (SEQ TD NO:2), FC / HV (SEQ ID NO:3), RGD (SEQ ID NO:4), FN, and Col IV were conducted using a modified Boyden chamber. Haptotactic migration was performed in triplicate and was assessed using a modified Boyden chamber (Corning, CoStar, Acton, Mass.). The assay was carried out as follows: the lower chamber was first blocked with 10% BSA for at least 30 minutes at 37° C. followed by 3 washings with PBS. The lower surface of the membrane on the upper chamber was coated with approximately 10 μL of protein or EMD peptide solution having a concentration between 500 ng / mL-300 μg / mL and allowed to incubate for 15-30 minutes at 37° C. and then allowed to air dry at room temperature under aseptic conditions. At least one assay was performed for each concentration. Basal cell media supplemented with 0.5% BSA was then added to the lower chamber an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com