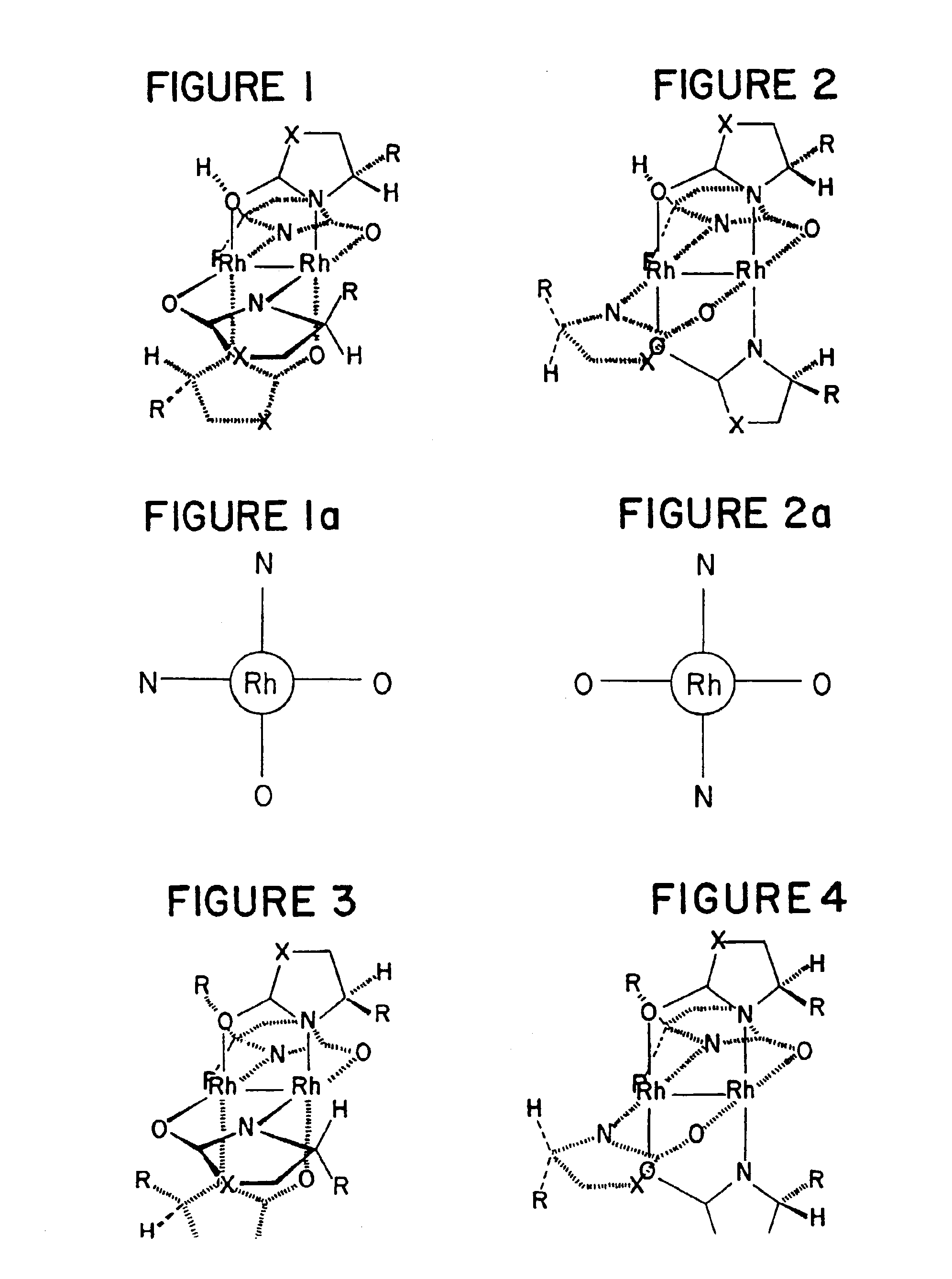
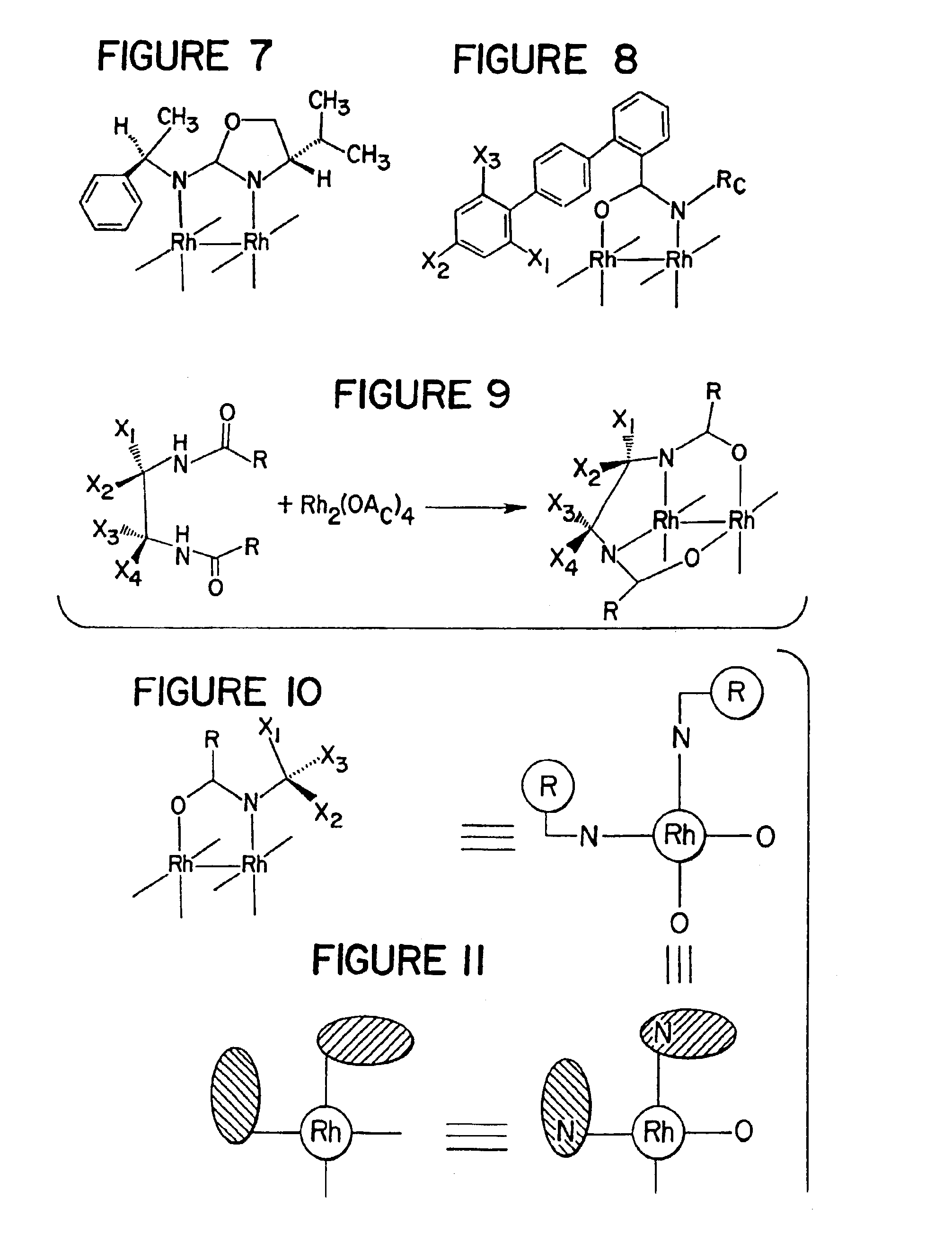
Method of enantioselectively catalyzing a reaction
a catalytic reaction and selective catalytic technology, applied in the field of catalytic methods, can solve the problems of low to moderate reported enantiomeric excesses of chiral copper catalysts, low stereoselectivities, and low product yield of tri-substituted olefins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
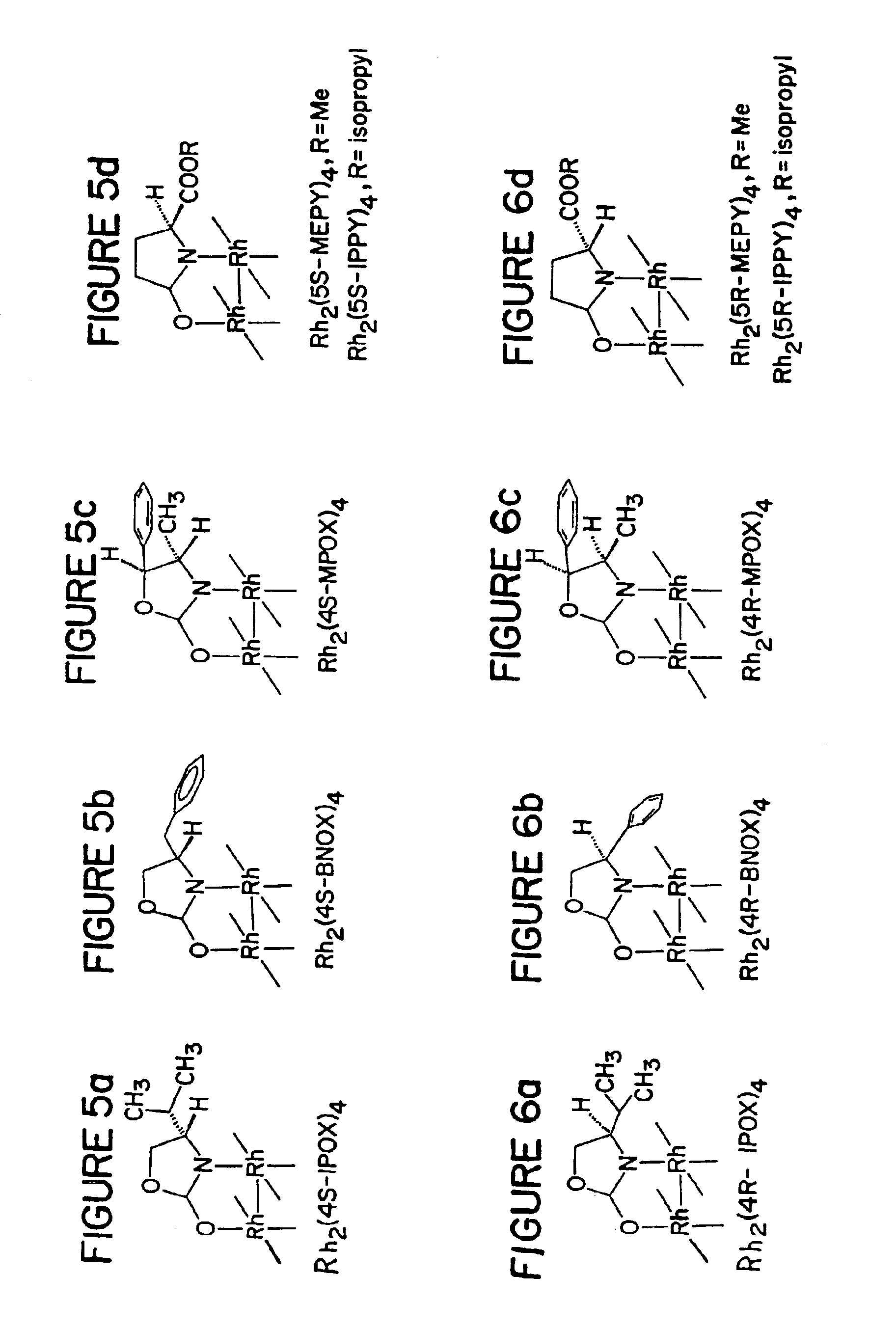
Preparation of catalyst Dirhodium(II) Tetrakis[(S)-(−)-4-benzyl-2-oxazolidinone](Rh2[4S-BNOX]4) (See FIG. 5b)
[0095]Rhodium(II) acetate (0.497 g, 1.12 mmol), prepared from rhodium trichloride according to the literature procedure (G. A. Rampel et al., Inorganic Synthesis. 13, 90 (1972)), and (S)-(−)-4-benzyl-2-oxazolidinone (2.40 g, 13.6 mmol) obtained from the Aldrich Chemical Company (29,464-0), in 50 mL of anhydrous chlorobenzene was refluxed under nitrogen in a Soxhlet extraction apparatus. The thimble was charged with a 3:1 mixture of sodium carbonate and sand which had been dried at 110° C. for 3 h, and a new thimble containing the sodium carbonate-sand mixture was introduced after refluxing for 24 h. After 49 h, as evidenced by HPLC analysis on a u-Bondapak-CN column, the dirhodium composite was >99% Rh2(4S-BNOX)4. Chlorobenzene was removed by distillation, and the resulting purple solid was chromatographed on a silica gel column using acetonitrile hexane (3:97 to 30:70) to se...
example 2
Preparation of catalyst Dirhodium(II) Tetrakis [(R)-(+)-4-benzyl-2-oxazolidinone](Rh2[4R-BNOX]4) (See FIG. 6b)
[0096]Rhodium(II) acetate (0.218g, 0.493 mmol) and (R)-(+)-4-benzyl-2-oxazolidinone (1.770 g, 10.0 mmol), from Fluka Chemical Company, in 50 mL of anhydrous chlorobenzene was refluxed under nitrogen for 39 h in a Soxhlet extraction apparatus according to the procedure in the previous example. Chromatographic separation of the purple solid, obtained after distillation of chlorobenzene, on a silica gel column, as previously described, yielded fractions that by HPLC analyses were >99.5% Rh2(4R-BNOX)4.
example 3
Preparation of catalyst dirhodium(II) tetrakis[(4S)-(−)-4-isopropyl-2-oxazolidinone](Rh2[4R-IPOX]4) (See FIG. 6a)
[0097]The subject catalyst was made in a procedure similar to that in Example 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific rotation | aaaaa | aaaaa |
| enantiomeric excess | aaaaa | aaaaa |
| specific rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com