Flexible thin layer open electrochemical cell
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
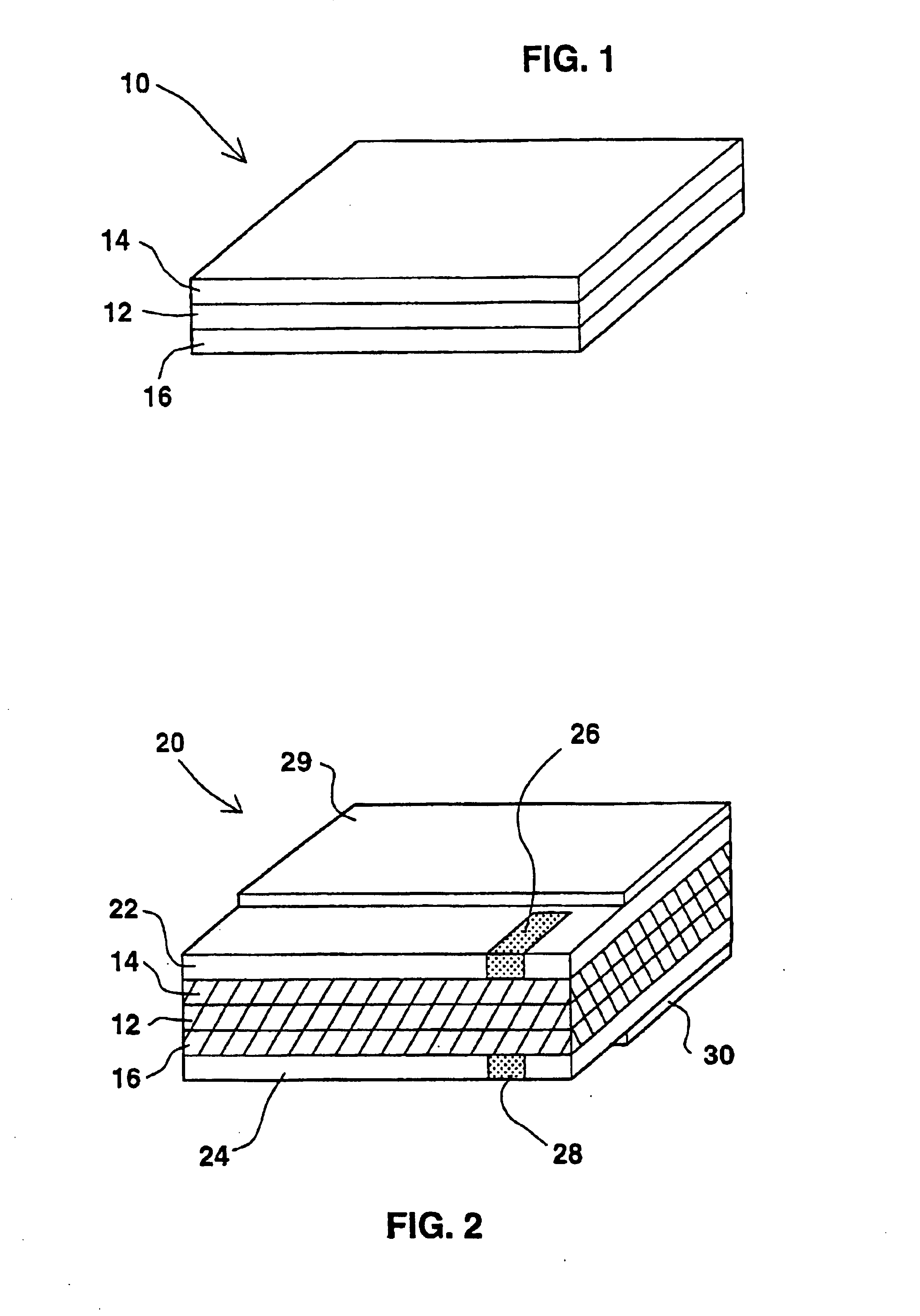
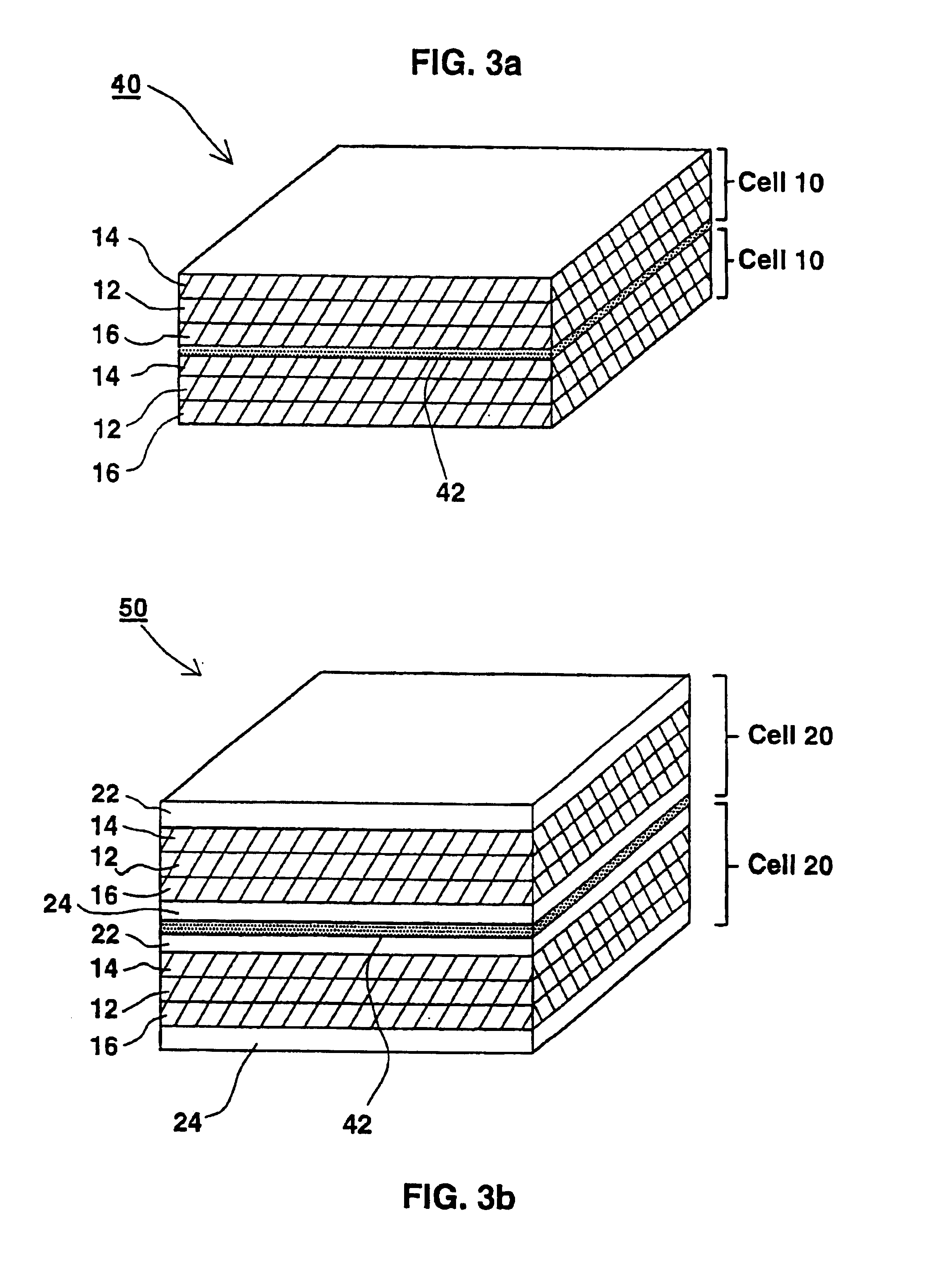
[0061]A solution containing 120 mg of polyvinylalcohol (an aqueous soluble polymer) and 1680 mg of zinc-chloride (a deliquescent material and an electroactive soluble material) in 1.2 ml of water was prepared. This solution had a glue like viscous appearance. A 4.5 cm×7 cm strip of a filter paper was thoroughly wetted with this solution by a printing or dipping technologies. A mixture of 300 mg zinc powder with the above solution was prepared and was printed on one side of the paper strip serving as the negative pole layer. On the other side printed was a mixture of 250 mg manganese-dioxide and 50 mg of a conductive carbon powder, together with the above solution, serving as the positive pole layer. When electrical contacts were made with both sides and were connected over a load an electrical current was measured. A current of 12 microampers per cm2 at a voltage of 1.7+1.2 volts was easily maintained for five days continuously under room conditions.
example 2
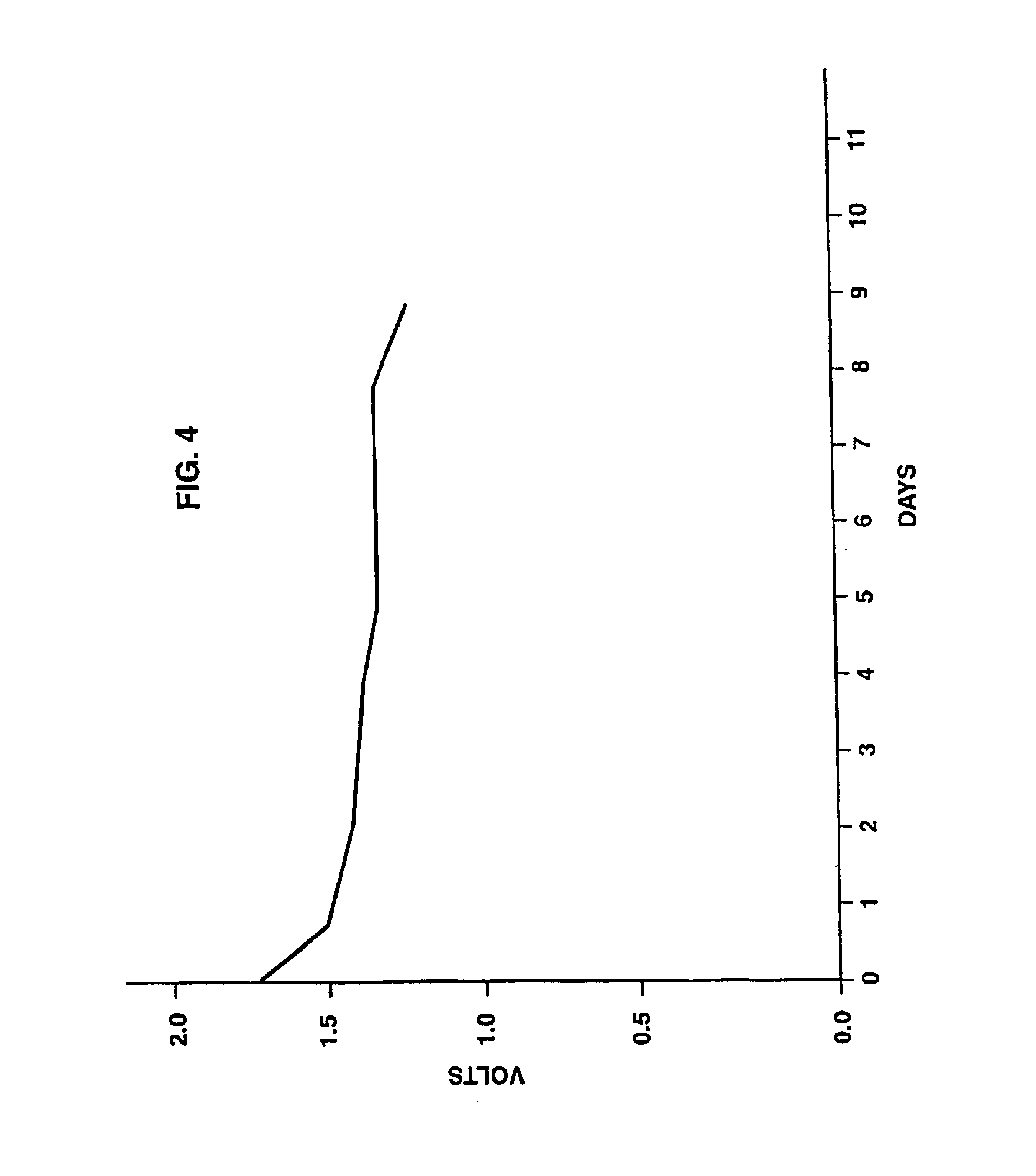
[0062]An open cell was prepared as described under Example 1 above and was connected to a voltmeter. As shown in FIG. 4, measurement of the voltage produced by the cell under room conditions revealed a pronounced voltage of 1.7+1.2 sustained for nine successive days.
example 3
[0063]A saturated potassium-hydroxide solution is prepared and brought to the viscosity of a glue by mixing with a water soluble polymer. A porous substance (e.g., a filter paper) is thoroughly wetted with this solution and a mixture of the solution with nickel-oxide powder is pasted on one side of the porous substance to form a positive pole layer and, a similar mixture with cadmium powder is pasted on the other side of the porous substance to form a negative pole layer. By connecting a voltmeter to the two sides a voltage of 1.2 volts is measured and a high current is measured when the two layers are contacted over a load. The cell does not dry out in the open and can be recharged if so desired.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Current | aaaaa | aaaaa |
| Electrical conductivity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com