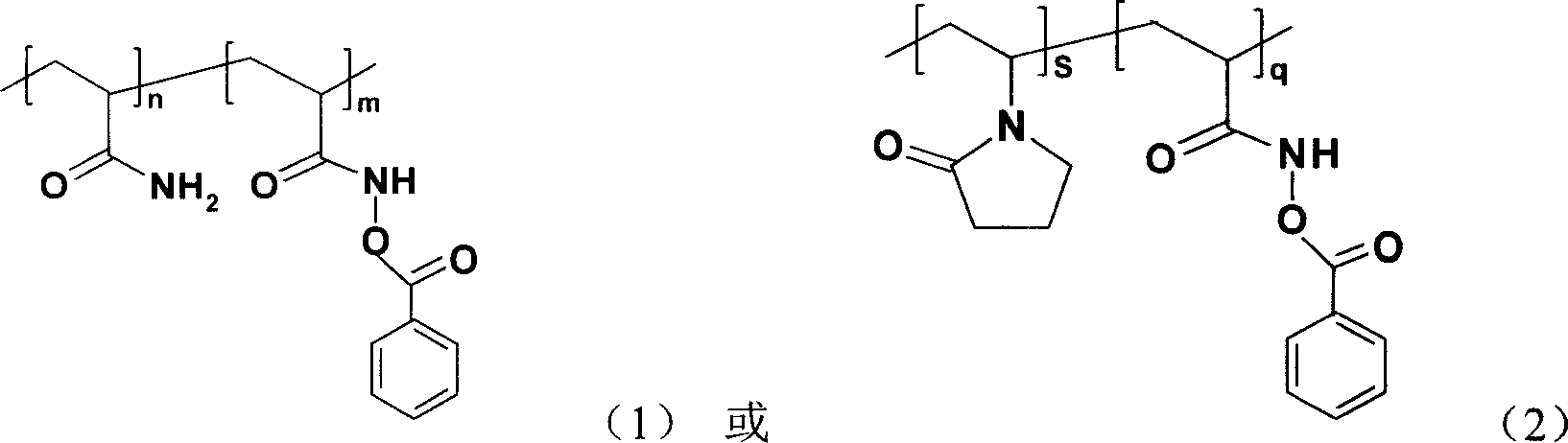
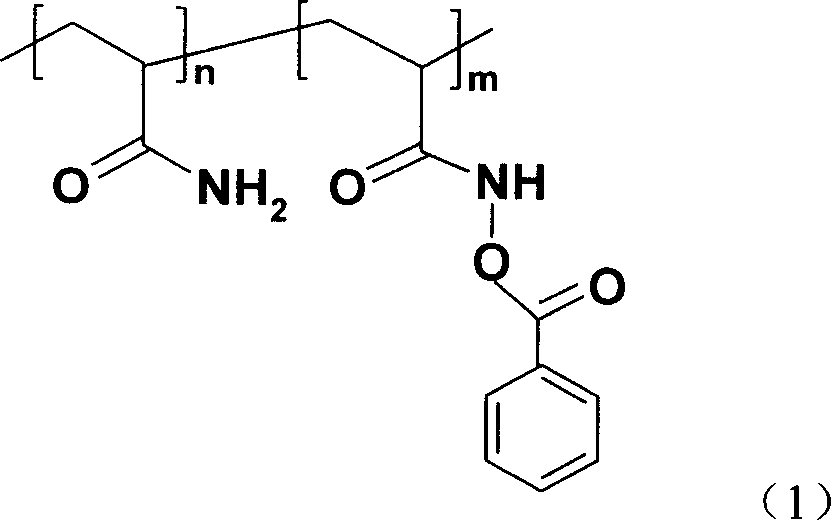
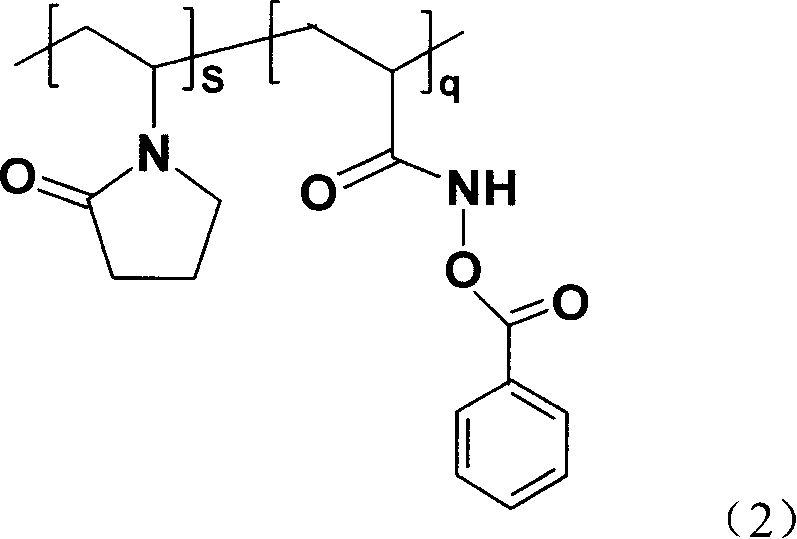
PH sensitive solution polyacrylic acyloxy oxo acetate and its synthesis method
A technology of polyacryloyl hydroxamate and synthesis method, applied in the field of novel carrier polylacenoyl hydroxamate and its synthesis, can solve the problem that the pH sensitivity of hydroxamate is rarely studied and the like, and achieve a good pH Sensitive erodibility, the effect of a simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] First take monomer AM 0.14mol and initiator AIBN 1.2×10 -3 mol, placed in a 250ml three-necked flask, completely dissolved with 45ml of absolute ethanol and 45ml of acetone, stirred and blown with nitrogen for 30min, then heated to 60°C. When white turbidity appeared, the reaction was continued for 2.5 h, and the reaction was stopped. Cool, filter with suction, wash 3 times with absolute ethanol, and vacuum-dry at 40°C until constant weight. Take 0.12mol NH 2 OH·HCl and 0.2mol NaOH were dissolved in 150ml of distilled water to obtain a free hydroxylamine solution, and 0.1mol PAM was slowly added to dissolve it completely, then reacted in a constant temperature water bath at 90°C for 6 hours to obtain an aqueous solution of hydroxamic acid. Then add 0.12mol NaOH to the reaction system for preparing hydroxamic acid, add an equivalent amount of freshly distilled benzoyl chloride dropwise to the solution under vigorous stirring, and after the product is fully separated, f...
Embodiment 2
[0024] First take monomer AM 0.14mol and initiator AIBN 1.2×10 -3 mol, placed in a 250ml three-necked flask, completely dissolved with 45ml of absolute ethanol and 45ml of acetone, stirred and blown with nitrogen for 30min, then heated to 60°C. When white turbidity appeared, the reaction was continued for 2.5 h, and the reaction was stopped. Cool, filter with suction, wash 3 times with absolute ethanol, and vacuum-dry at 40°C until constant weight. Take 0.08mol NH 2 Dissolve OH·HCl and 0.15mol NaOH in 150ml distilled water to obtain free hydroxylamine solution, slowly add 0.1mol PAM, after complete dissolution, react in a constant temperature water bath at 90°C for 6 hours to obtain an aqueous solution of hydroxamic acid. Then add 0.08mol NaOH to the reaction system for preparing hydroxamic acid, add an equivalent amount of freshly distilled benzoyl chloride dropwise to the solution under vigorous stirring, and after the product is fully separated, centrifuge, wash with dist...
Embodiment 3
[0026] First, take monomer AM 0.14mol and initiator AIBN 1.2×10 -3 mol, placed in a 250ml three-necked flask, completely dissolved with 45ml of absolute ethanol and 45ml of acetone, stirred and blown with nitrogen for 30min, then heated to 60°C. When white turbidity appeared, the reaction was continued for 2.5 h, and the reaction was stopped. Cool, filter with suction, wash 3 times with absolute ethanol, and vacuum-dry at 40°C until constant weight. Take 0.05mol NH 2 OH·HCl and 0.12mol NaOH were dissolved in 150ml of distilled water to obtain a free hydroxylamine solution, and 0.1mol PAM was slowly added to dissolve it completely, then reacted in a constant temperature water bath at 90°C for 6 hours to obtain an aqueous solution of hydroxamic acid. Then add 0.05mol NaOH to the reaction system for preparing hydroxamic acid, add an equivalent amount of freshly distilled benzoyl chloride dropwise to the solution under vigorous stirring, and after the product is fully separated,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com