Process for preparing green light emitting materials polyalkyl fluorene
A technology of emitting material and polyalkyl fluorene, which is applied in the fields of organic polymer photoelectric functional materials, organic conductors, and organic semiconductors, can solve problems such as reducing the luminous efficiency of materials, and achieve the effects of simplifying preparation technology, reducing costs, and improving product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
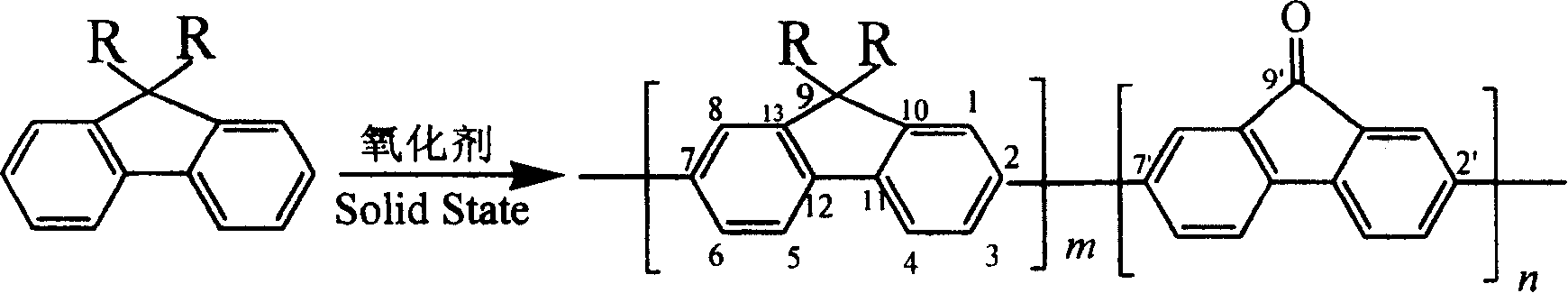
[0026] Weigh 0.67 g of 9,9-dihexylfluorene and 0.33 g of anhydrous ferric chloride into a mortar, mix them uniformly, and grind at 20°C. After grinding for 20 minutes, add 0.33 g of anhydrous ferric chloride to continue grinding. After 20 minutes, 0.33 g of anhydrous ferric chloride was added again for grinding. The reaction was stopped after a total of 1 hour of trituration. Wash the product in the mortar with industrial methanol, and separate the solid and liquid by centrifugation to remove ferric chloride and unreacted monomers. After repeated washing, the product is dried in a constant temperature oven (50°C). After drying, dissolve the product completely with a small amount of chloroform, add methanol with a volume four to five times the amount of chloroform to precipitate the polymer, and centrifuge again to separate the solid from the liquid. Then the solid product was extracted with methanol for 24 hours in a Soxhlet extractor so that the amount of residual ferric c...
Embodiment 2
[0029] Weigh 0.56 g of dibutylfluorene and 0.33 g of anhydrous ferric chloride into a mortar, mix them evenly, and grind at 20°C. After grinding for 20 minutes, add 0.33 g of anhydrous ferric trichloride and mix with the original reactant, and continue grinding. After 20 minutes, add 0.33 g of anhydrous ferric trichloride again and grind. The reaction was stopped after a total of 1 hour of trituration. Wash the product in the mortar with industrial ethanol, and separate the solid and liquid by centrifugation to remove ferric chloride and unreacted monomers. After repeated washing, the product is dried in a constant temperature oven (50°C). After drying, dissolve the product completely with a small amount of chloroform, add ethanol with a volume four to five times the amount of chloroform to precipitate the polymer, and centrifuge again to separate the solid from the liquid. The solid product is then extracted with methanol for 24 hours in a Soxhlet extractor to reduce the r...
Embodiment 3
[0031]Weigh 0.44 g of diethylfluorene and 0.33 g of anhydrous ferric chloride into a mortar, mix them evenly, and grind at 20°C. After grinding for 20 minutes, add 0.33 g of anhydrous ferric chloride again and mix with the original reactant, continue grinding for 20 minutes, add 0.33 g of anhydrous ferric chloride again, and grind. The reaction was stopped after a total of 1 hour of trituration. Wash the product in the mortar with industrial ethanol, and separate the solid and liquid by centrifugation to remove ferric chloride and unreacted monomers. After repeated washing, the product is dried in a constant temperature oven (50°C). After drying, the product is completely dissolved with a small amount of tetrahydrofuran, and the polymer is precipitated by adding ethanol whose volume is four to five times the amount of tetrahydrofuran, and centrifuged again to separate the solid from the liquid. The solid product is then extracted with methanol for 24 hours in a Soxhlet extra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| decomposition temperature | aaaaa | aaaaa |
| emission peak | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com