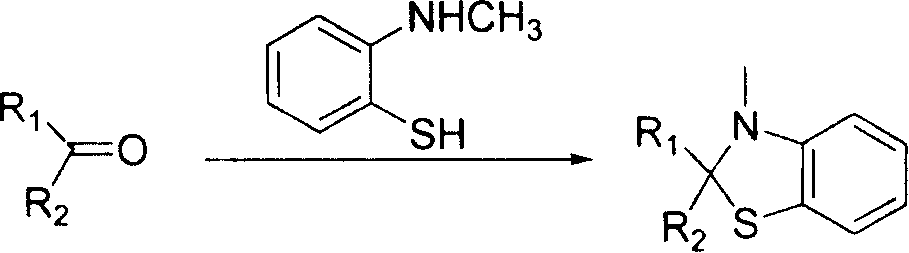
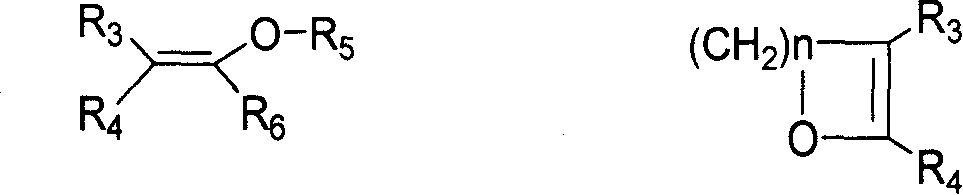Method for removing carbonylazathia acetal protection group
A technology of heteroketal and carbonyl nitrogen, which is applied in the field of deprotection of carbonyl protecting groups, can solve the problems of mercury chloride toxicity, complex reaction behavior, and inability to apply industrial production, and achieve mild reaction conditions, cheap and easy-to-obtain reagents, and reaction solution cleaning effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 117
[0029] Example 1 17α-Acetoxy-17β-cyano-androst-1,4,9(11)-trien-3-one
[0030] 1.06mmol 3'-methylspiro[androst-1,4,9(11)-triene-3,2'(3'H)benzothiazole]-17β-cyano-17α-acetate, 10mL Dissolve the organic solvent, add 1.06mmol p-toluenesulfonic acid, measure the pH as 1, add 10.6mmol CH 2 =CH-OC 2 h 5 , stirred at room temperature for 15min, stopped the reaction, diluted with ethyl acetate, washed three times with aqueous sodium carbonate solution, back pumped the aqueous layer twice, combined the organic phases, and anhydrous Na 2 SO 4 Drying, filtration, concentration, ethyl acetate gave yellow crystals, yield: 91.07%, mp: 206-208 ° C, identified as 17α-acetoxy-17β-cyano-androst-1,4,9 (11 )-trien-3-one.
[0031] The mixed sample of the mother liquor was applied to a silica gel column, and eluted with petroleum ether: ethyl acetate / 100:1 to obtain 137.9 mg of a colorless oil, which was identified as 2-(1-ethoxy-ethylthio)-N-methyl- aniline.
[0032] The product 2-(1-ethoxyl...
Embodiment 2
[0033] Example 2 17α-Acetoxy-17β-cyano-androst-1,4,9(11)-trien-3-one
[0034] 1.06mmol 3'-methylspiro[androst-1,4,9(11)-triene-3,2'(3'H)benzothiazole]-17β-cyano-17α-acetate, 10mL Dissolve the organic solvent, add 1.06mmol benzenesulfonic acid, measure the pH as 1, add 10.6mmol CH 2 =CH-OC 4 h 9 , stirred at room temperature for 15min, stopped the reaction, diluted with ethyl acetate, washed three times with sodium hydroxide solution, back pumped the water layer twice, combined the organic phases, anhydrous Na 2 SO 4 Drying, filtration, concentration, ethyl acetate gave yellow crystals, yield: 93.66%, mp: 205-207 ° C, identified as 17α-acetoxy-17β-cyano-androst-1,4,9 (11 )-trien-3-one.
[0035] The mixed sample of the mother liquor was applied to a silica gel column, and eluted with petroleum ether: ethyl acetate / 100:1 to obtain 175.7 mg of a colorless oil, which was identified as 2-(1-butoxy-ethylthio)-N-methyl- aniline.
Embodiment 3
[0036] Example 3 17α-Acetoxy-17β-cyano-androst-1,4,9(11)-trien-3-one
[0037] 1.06mmol 3'-methylspiro[androst-1,4,9(11)-triene-3,2'(3'H)benzothiazole]-17β-cyano-17α-acetate, 10mL Dissolve the organic solvent, add 1.06mmol methanesulfonic acid, measure the pH to 1, add 10.6mmol dihydropyran, stir at room temperature for 20min, stop the reaction, dilute with ethyl acetate, wash three times with triethylamine aqueous solution, and back pump the water layer twice, Combined organic phases, anhydrous Na 2 SO 4 Drying, filtration, concentration, ethyl acetate gave yellow crystals, yield: 92.87%, mp: 206-207 ° C, identified as 17α-acetoxy-17β-cyano-androst-1,4,9 (11 )-trien-3-one.
[0038] The mixed sample of the mother liquor was applied to a silica gel column, and eluted with petroleum ether: ethyl acetate / 100:1 to obtain 166.4 mg of a colorless oil, which was identified as 2-(2-tetrahydropyranyl-sulfanyl)-N-methyl -aniline.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com