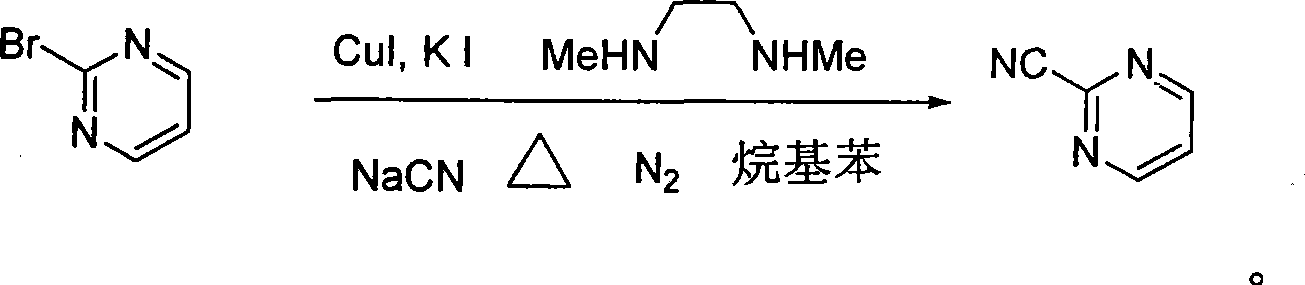
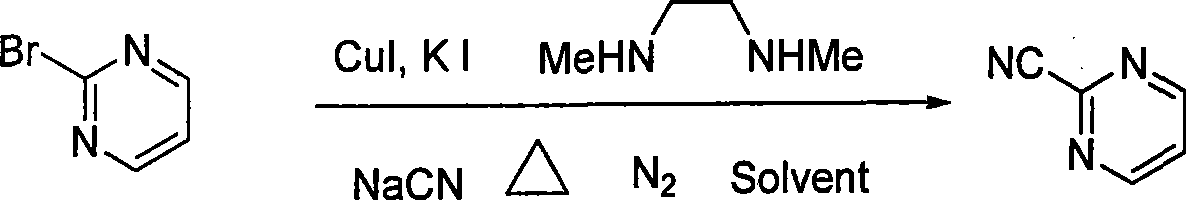
Preparation process of key intermediate 2-cyano pyrimidine of bosentan
A cyanopyrimidine and preparation process technology, which is applied in the field of preparation of 2-cyanopyrimidine, the key intermediate of bosentan medicine, can solve the problems of complex operation, low yield, long operation period, etc., and achieve simple feeding and post-processing Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] In a 1-liter three-necked flask, 500 milliliters of toluene, 48 grams (0.3 moles) of 2-bromopyrimidine, 11.8 grams (0.36 moles, 1.2 equivalents) of sodium cyanide, 5.73 grams (30 mmoles, 0.1 equivalents) of sodium cyanide were added successively under nitrogen protection. ) cuprous iodide, 10 grams of potassium iodide (60 millimoles, 0.2 equivalents), 26.4 grams of N, N'-dimethylethylenediamine (0.3 moles, 1.0 equivalents), stirred and reacted at 110 ° C for 30 hours under nitrogen protection , end the reaction, then filter, wash the filtrate with water, dry with anhydrous sodium sulfate, filter, concentrate, and recrystallize with petroleum ether to obtain white needle-shaped crystals of 2-cyanopyrimidine, with a yield of 71%, a purity of 97%, and a melting point of 41- 43°C.
Embodiment 2
[0021] In a 1-liter three-necked flask, 500 milliliters of ethylbenzene, 48 grams (0.3 moles) of 2-bromopyrimidine, 11.8 grams (0.36 moles, 1.2 equivalents) of sodium cyanide, 5.73 grams (30 millimoles, 0.1 Equivalent) cuprous iodide, 10 grams of potassium iodide (60 millimoles, 0.2 equivalents), 26.4 grams of N, N'-dimethylethylenediamine (0.3 moles, 1.0 equivalents), stirred reaction at 120 ° C under nitrogen protection for 25 hours, the reaction was terminated, followed by filtration, the filtrate was washed with water, dried with anhydrous sodium sulfate, filtered, concentrated, and recrystallized with petroleum ether to obtain white needle-like crystals of 2-cyanopyrimidine, with a yield of 75%, a purity of 98%, and a melting point of 42 ~43°C.
Embodiment 3
[0023] In a 1-liter three-necked flask, 500 milliliters of toluene, 48 grams (0.3 moles) of 2-bromopyrimidine, 11.8 grams (0.36 moles, 1.2 equivalents) of sodium cyanide, 5.73 grams (30 mmoles, 0.1 equivalents) of sodium cyanide were added successively under nitrogen protection. ) cuprous iodide, 11 grams of potassium iodide (66 millimoles, 0.22 equivalents), 26.4 grams of N, N'-dimethylethylenediamine (0.3 moles, 1.0 equivalents), stirred and reacted at 105 ° C for 36 hours under nitrogen protection , finish the reaction, then filter, wash the filtrate with water, dry with anhydrous sodium sulfate, filter, concentrate, and recrystallize with sherwood oil to obtain white needle-like crystals of 2-cyanopyrimidine, with a yield of 65%, a purity of 98%, and a melting point of 40~ 42°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com