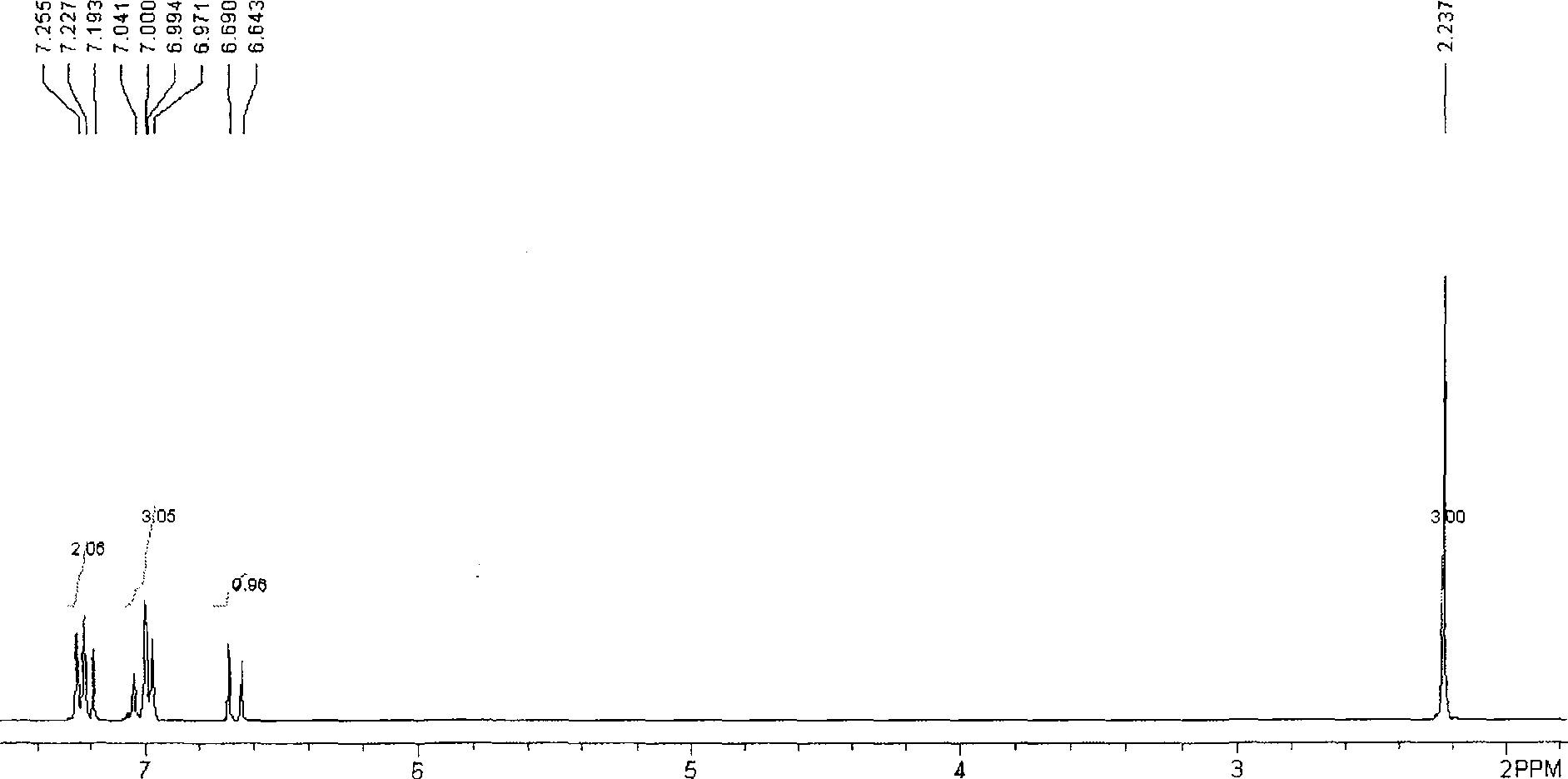
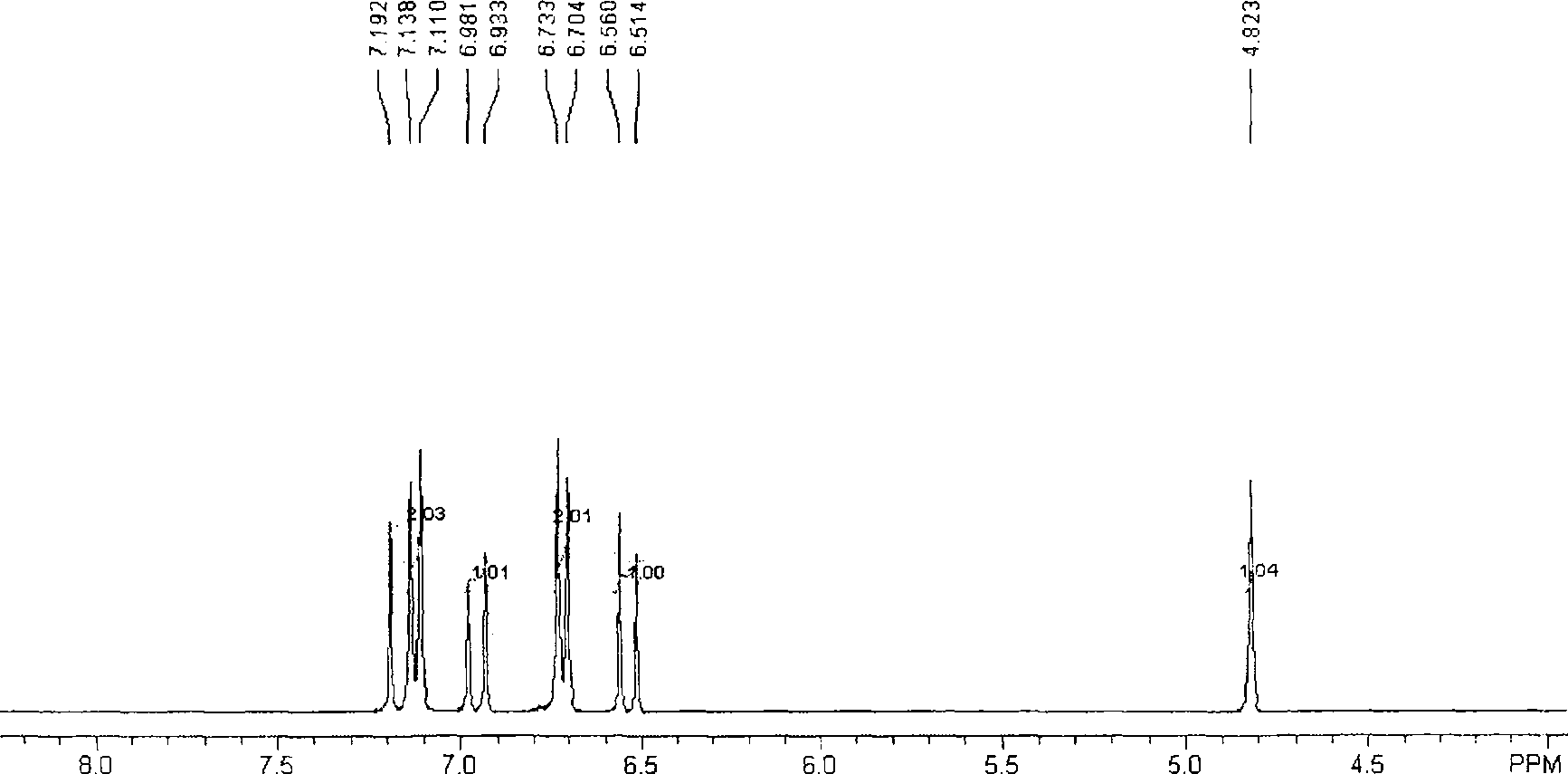
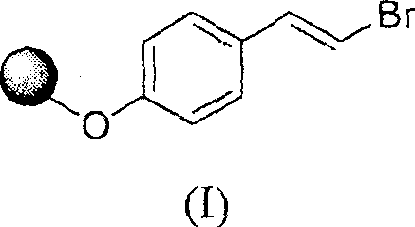
Method for preparing trans-4-(beta-bromoethyl) phenoxy-benzylic resin (I)
A technology of phenoxybenzyl type and vinyl bromide, which is applied in the field of preparation of trans-4-phenoxybenzyl type resin, and achieves the effect of great application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] (1) Preparation of 4-hydroxycinnamic acid (II)
[0039]
[0040] In a 50mL flask equipped with a reflux condenser and a thermometer, add 0.611g (5mmol) of 4-hydroxybenzaldehyde, 1.040g (10mmol) of malonic acid, 10mL of pyridine, and 0.01mL of piperidine, raise the temperature to 80°C, and stir the reaction 6 to 8 hours, followed by TLC detection (ethyl acetate: n-hexane: acetic acid = 10:20:1 as developer). After the reaction, cool to room temperature, neutralize with 6M cold hydrochloric acid to pH = 2, filter, wash with ice water and ethyl acetate, P 2 o 5 Vacuum drying gave 0.700 g of light yellow powder, namely 4-hydroxycinnamic acid (II), with a yield of 92%.
[0041] (2) Preparation of trans-(4-acetoxy) cinnamic acid (III)
[0042]
[0043] In a 25mL flask equipped with a calcium chloride drying tube, add 0.761g (5mmol) 4-hydroxycinnamic acid (II), 5mL acetic anhydride, 0.5mL triethylamine, react at 70°C for 2 hours, TLC tracking detection (acetic acid Eth...
Embodiment 2
[0058] (1) Preparation of trans-(4-acetoxy) cinnamic acid (III)
[0059]
[0060] In a 25mL flask equipped with a calcium chloride drying tube, add 0.761g (5mmol) of commercially available 4-hydroxycinnamic acid (II), 5mL of acetic anhydride, and 0.7mL of triethylamine, and react at 80°C for 3 hours, followed by TLC Detection (ethyl acetate: n-hexane: acetic acid=10:20:1 as developer). After the reaction, add 15 mL of water, and stir vigorously at room temperature for 0.5 hour to completely decompose the acetic anhydride and the system becomes cloudy. Filtered, the filter cake was washed 3 times with 20mL water, washed with P 2 o 5 After vacuum drying, 0.979 g of trans-(4-acetoxy)cinnamic acid (III) was obtained, with a yield of 95%.
[0061] (2) Preparation of trans-4-(β-bromovinyl)phenyl acetate (IV)
[0062]
[0063] To 10 mL of reaction medium (9.0 mL of acetonitrile and 1.0 mL of water) was added 1.031 g (5.0 mmol) of trans-(4-acetoxy)cinnamic acid (III), 0.934 ...
Embodiment 3
[0071] (1) Preparation of trans-(4-acetoxy) cinnamic acid (III)
[0072]
[0073] In a 25mL flask equipped with a calcium chloride drying tube, add 0.761g (5mmol) of commercially available 4-hydroxycinnamic acid (II), 8mL of acetic anhydride, and 0.7mL of triethylamine, and react at 60°C for 2 hours, followed by TLC Detection (ethyl acetate: n-hexane: acetic acid=10:20:1 as developer). After the reaction was completed, 15 mL of water was added, and vigorously stirred at room temperature for 0.5 hour, so that the acetic anhydride was completely decomposed and the system became cloudy. Filtered, the filter cake was washed 3 times with 20mL water, washed with P 2 o 5 After vacuum drying, 1.0 g of trans-(4-acetoxy)cinnamic acid (III) was obtained with a yield of 97%.
[0074] (2) Preparation of trans-4-(β-bromovinyl)phenyl acetate (IV)
[0075]
[0076] In 10 mL of reaction medium (9.5 mL of acetonitrile and 0.5 mL of water) were added 1.031 g (5.0 mmol) of trans-(4-acet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com