Method for producing swine fever live vaccine with cell line
A live swine fever vaccine and cell line technology, which can be used in pharmaceutical formulations, medical preparations containing active ingredients, antibody medical ingredients, etc., can solve the difficulties in improving vaccine production and efficacy, exogenous virus contamination of cells, and toxin-producing drops It can achieve good economic benefits and application prospects, high virus content, and solve the effect of exogenous pathogen contamination of cattle.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
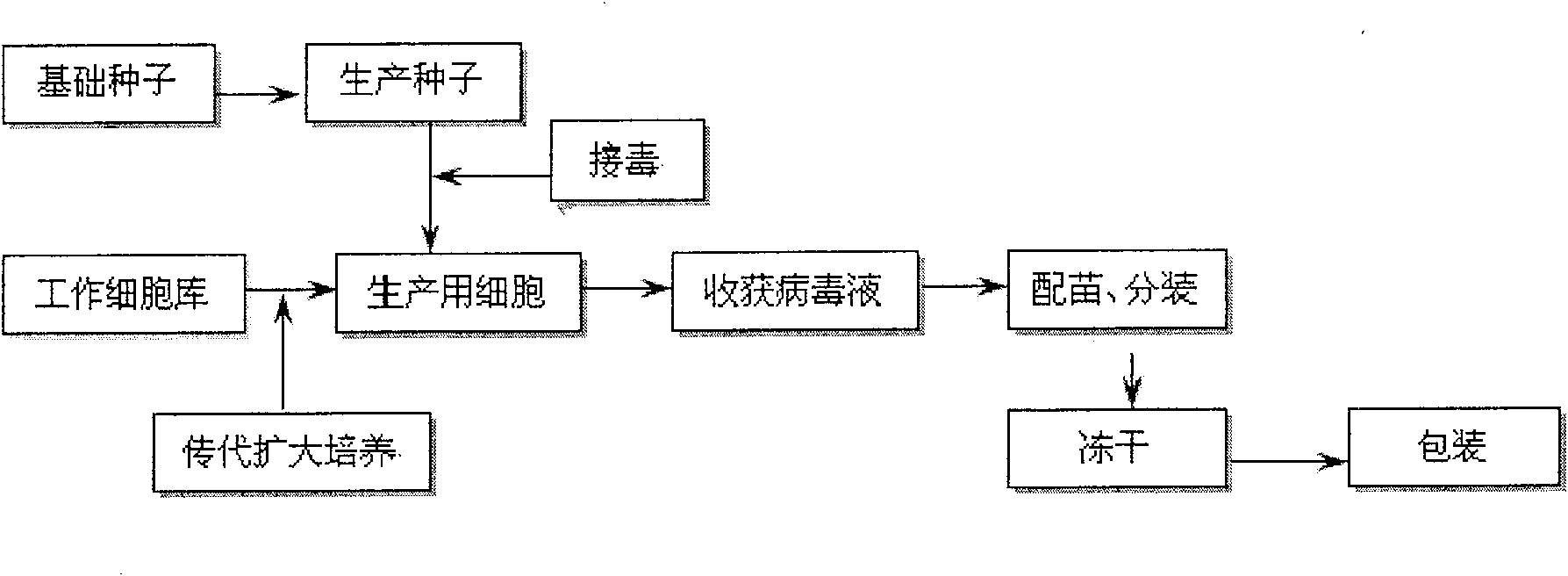
[0027] (1) Selection of cells for seedling production: use porcine testis (ST) cell line;
[0028] (2) Subculture and culture of cells for seedling production: ST cell line is digested and subcultured with EDTA-trypsin cell dispersion, then cultured with cell growth solution at 36°C to form a good monolayer, used for continued subculture or virus inoculation;
[0029] (3) Propagation of cytotoxic species: Use cell maintenance solution to prepare a 0.3% virus suspension from fresh spleen venom, inoculate a well-growing ST cell line monolayer, and continue culturing at 36°C. Harvest and change the liquid every 4 days, and take the secondary and tertiary cell culture venom as the production poison;
[0030] Identification of cytotoxic species: The identification of cytotoxic species is in full compliance with the standard of attenuated strains of classical swine fever lapinization and has no side effects on pigs. The cytotoxic species contains more than 500,000 rabbit infections per 1 m...
Embodiment 2
[0038] (1) Selection of cells for seedling production: use pig kidney (PK15) cell line;
[0039] (2) Subculture and culture of cells for seedling production: PK15 cell line is digested and subcultured with EDTA-trypsin cell dispersion, then cultured with cell growth solution at 37°C to form a good monolayer, used for continued subculture or virus inoculation;
[0040] (3) Propagation of cytotoxic species: Using cell maintenance solution, fresh spleen toxin was made into a 0.4% virus suspension, inoculated with a monolayer of well-growing PK15 cell line, and kept at 37°C to continue the culture. Harvest and change the liquid every 5 days, and take the secondary and tertiary cell culture venom as the production poison;
[0041] Identification of cytotoxic species: The identification of cytotoxic species is in full compliance with the standard of attenuated strains of classical swine fever lapinization and has no side effects on pigs. The cytotoxic species contains more than 500,000 rab...
Embodiment 3
[0049] (1) Selection of cells for seedling production: use pig kidney (IBRS-2) cell line;
[0050] (2) Subculture and culture of cells for seedling production: IBRS-2 cell line is digested and subcultured with EDTA-trypsin cell dispersion, then cultured with cell growth medium at 37°C to form a good monolayer, used to continue subculture or inoculation virus;
[0051] (3) Propagation of cytotoxic species: Use cell maintenance solution to make 0.5% virus suspension from fresh spleen venom, inoculate a monolayer of well-growing IBRS-2 cell line, and continue culturing at 37°C. Harvest and change the liquid every 4 days, and take the secondary and tertiary cell culture venom as the production poison;
[0052] Identification of cytotoxic species: The identification of cytotoxic species is in full compliance with the standard of attenuated strains of classical swine fever lapinization and has no side effects on pigs. The cytotoxic species contains more than 500,000 rabbit infections per ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com