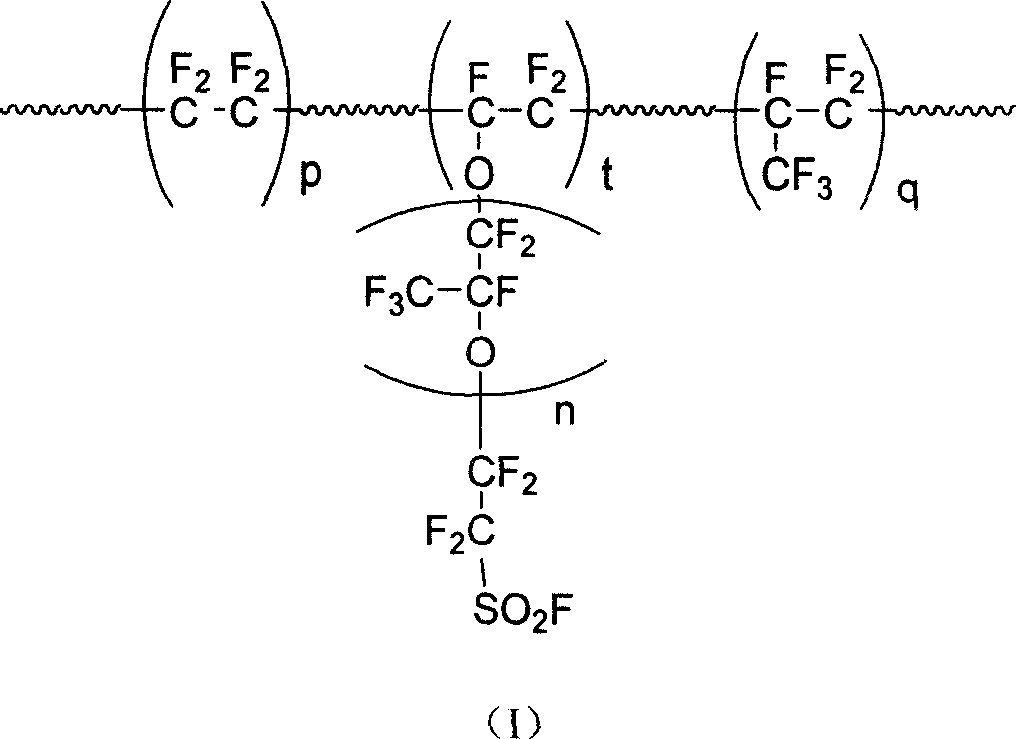
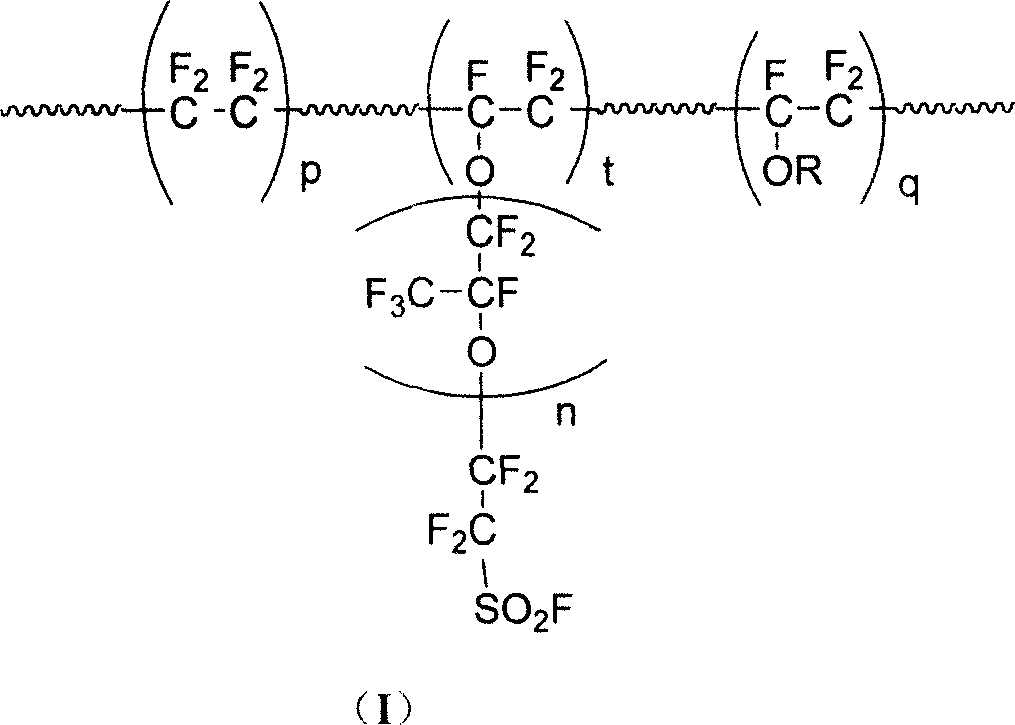
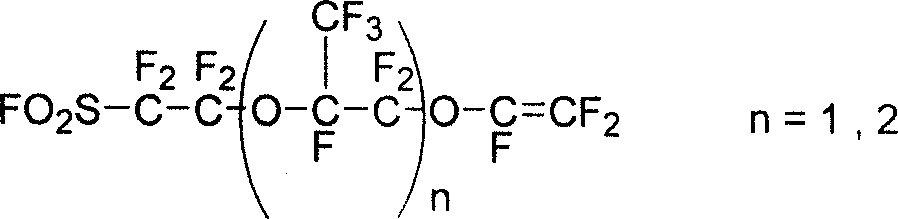Perfluoro - resin with ion exchange, and application
A technology of ion exchange and ion exchange capacity, applied in the field of perfluororesin and its application as ion exchange fiber material, to increase the effective area, reduce membrane resistance and cell voltage, and solve technical problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Perfluorosulfonyl vinyl ether (n=2, molecular weight is 612g / mol), molecular formula is:
[0030]
[0031] The synthesis method is as follows:
[0032] Tetrafluoroethylene and liquid SO 3 Reaction to prepare sultone, the purified sultone product is catalyzed by a composite fluoride ion catalyst and reacted with hexafluoroepoxy to prepare a perfluoroacyl fluoride product with a sulfonyl fluoride end group, which has a sulfonyl fluoride end group Perfluoroalkene ether compounds can be prepared by cracking and decarboxylation of perfluoro compounds at high temperature.
Embodiment 2
[0034] Add 23.2g perfluoroalkyl ether containing melamine structure and 185g distilled water as dispersant / solvent in 1L high-pressure stainless steel reaction kettle equipped with stirring, heating and nitrogen feed device, perfluorosulfonyl vinyl ether 5.3g (n =2) and 0.2g of benzoyl peroxide, replace the air in the reactor with high-purity nitrogen, feed 92.8g of tetrafluoroethylene and 1.4g of perfluoromethyl vinyl ether, raise the temperature to 80°C and stir for 3h, and the reaction ends After cooling, deflate to make the pressure inside the kettle become normal pressure, release the product from the discharge pipe, precipitate in a large amount of water, filter to obtain a powder product, wash with distilled water several times, and dry under vacuum at 100°C. Dispersant and water phase, liquid separation and recycling.
[0035] The prepared polymer material perfluororesin shows by fluorine NMR test that the weight percentage of perfluorosulfonyl vinyl ether is 4.89%, th...
Embodiment 3
[0037] Add 23.2g perfluoroalkyl ether containing melamine structure and 185g distilled water as dispersant / solvent in the 1L high-pressure stainless steel reactor that is equipped with stirring, heating, nitrogen feed device, perfluorosulfonyl vinyl ether 6.3g (n =2) and 0.2g of benzoyl peroxide, replace the air in the reactor with high-purity nitrogen, feed 102.8g of tetrafluoroethylene and 1.84g of perfluoropropyl vinyl ether, raise the temperature to 80°C and stir for 3h, and the reaction ends After cooling, deflate to make the pressure inside the kettle become normal pressure, release the product from the discharge pipe, precipitate in a large amount of water, filter to obtain a powder product, wash with distilled water several times, and dry under vacuum at 100°C. Dispersant and water phase, liquid separation and recycling.
[0038] The prepared polymer material perfluororesin shows that its thermal decomposition temperature is 371° C. by DTA test, and its melting tempera...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melt index | aaaaa | aaaaa |
| Thermal decomposition temperature | aaaaa | aaaaa |
| Melting temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com