Anti tumor translocation peptide of scorpion, preparation method and application
An anti-tumor metastasis, scorpion venom gland technology, applied in anti-tumor drugs, botanical equipment and methods, biochemical equipment and methods, etc., can solve the problem of low yield and achieve the effect of inhibiting chloride ion channels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Example 1: Design of primers and PCR amplification of scorpion anti-tumor metastasis peptide gene
[0073] Design PCR primers according to the sequence of the scorpion anti-tumor metastasis peptide gene (BmKCTL gene) provided by SEQID NO: 1, for example: A1, 5'-GCCGGATCCCCGATGACGATGACAAGTGTGGGCCTTGCTTTAC-3', A2: 5'-GCCCTCGAGTCATTCACGGTTACACAGACATTG-3', to obtain from scorpion venom gland cells The clone of the BmKCTL gene (SEQID NO: 1) obtained by screening in the cDNA library was used as a template to carry out PCR reaction to amplify the scorpion anti-tumor metastasis peptide gene in large quantities. PCR reaction conditions: 1 μl Taq polymerase (1U), 0.5 μl four (adenine, guanine, cytosine, thymine) deoxymononucleotide mixture (10mmol / L), 16.5 μl sterile double distilled Water, 2.5 μl 10-fold PCR buffer, 1.5 μl magnesium chloride (25 mmol / L), A1 (10 μmol / L) and A2 (10 μmol / L) primers, and 1 μl each of BmKCTL gene template in a total volume of 25 μl. PCR reaction pro...
Embodiment 2
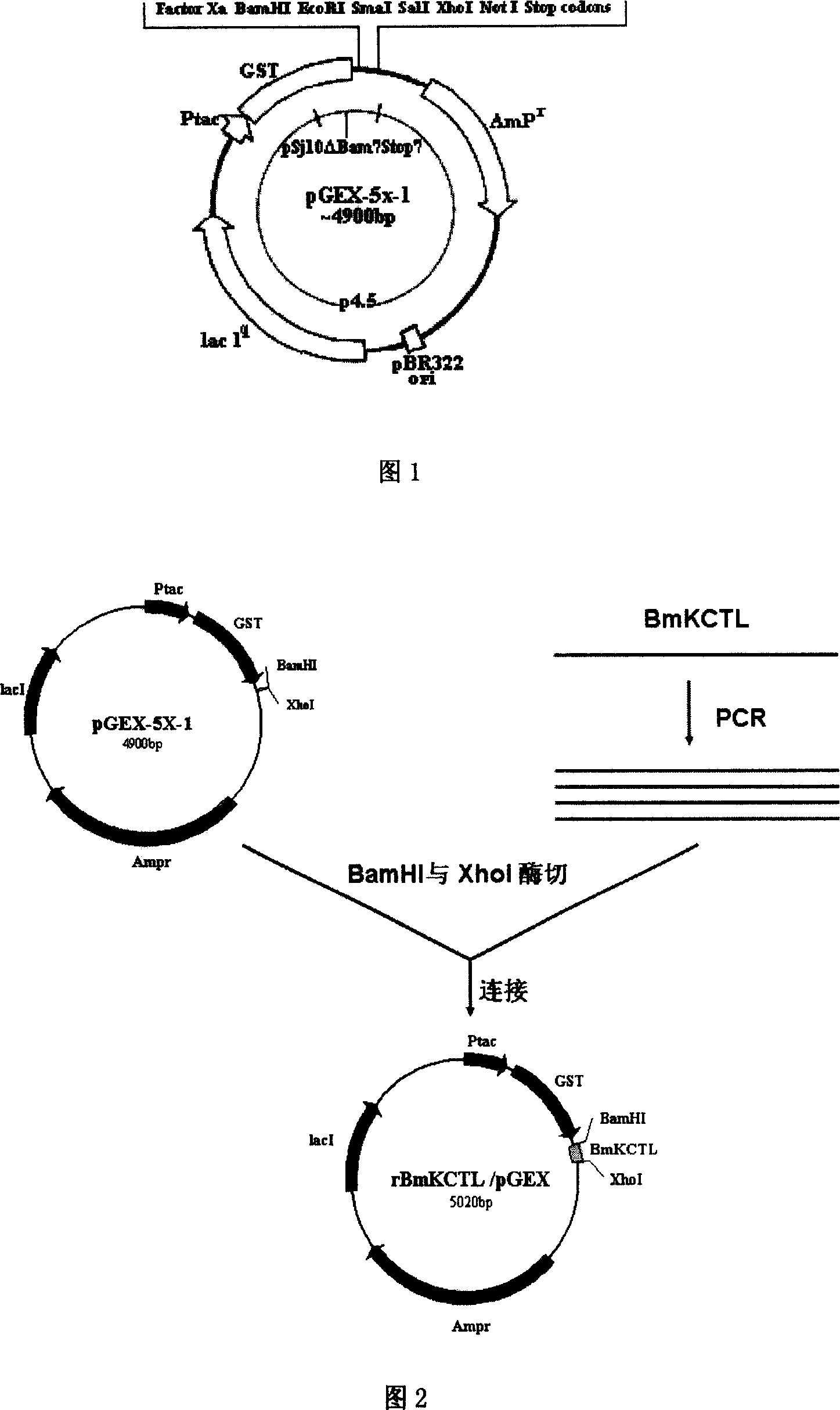
[0074] Embodiment 2: Construction of recombinant expression vector (rBmKCTL / pGEX)
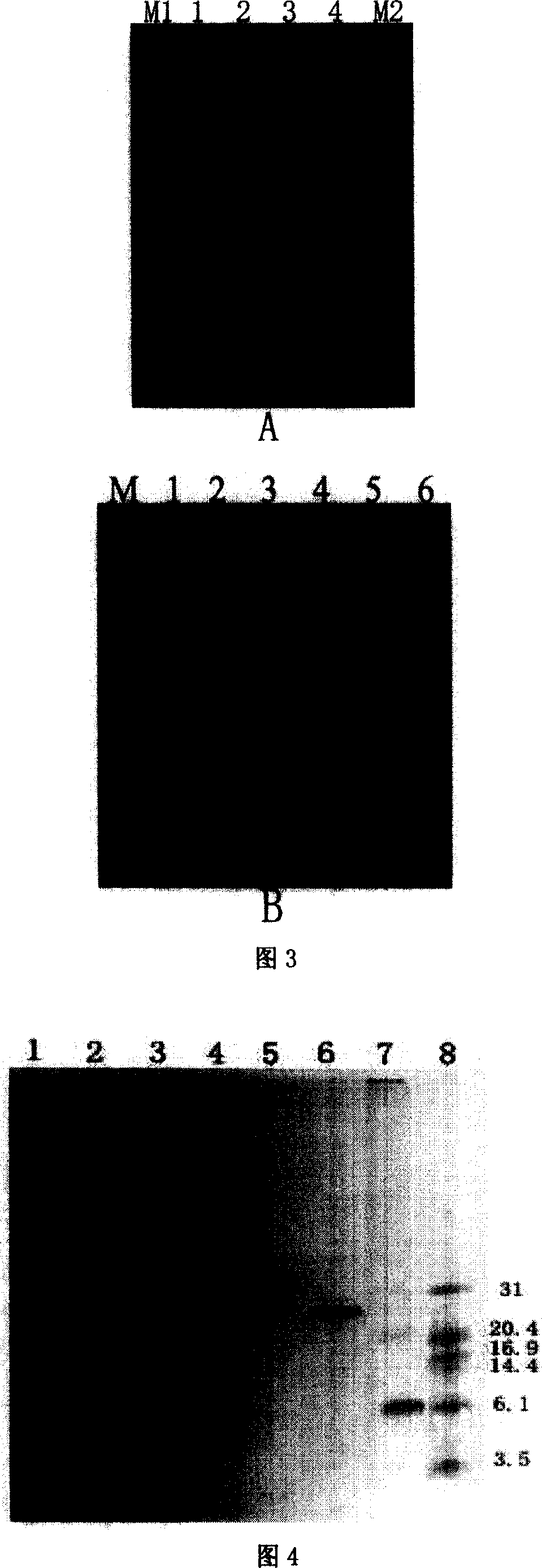
[0075] The PCR product obtained in Example 1 was extracted with restriction endonucleases through phenol: chloroform: isopentyl glycol (25: 24: 1), precipitated with absolute ethanol (2.5 times volume) and washed with 50 μl TE buffer (recorded After this example) to dissolve the precipitate. The recovered PCR product and expression vector pGEX-5x-1 plasmid were digested with restriction endonucleases Bam HI and Xho I (products of Takara Company). Enzyme digestion reaction: 1 μl each of BamHI (14U / μl) and XhoI (20U / μl), 2.5 μl of 10-fold buffer, 50-100 ng of PCR product or pGEX-5x-1 plasmid, add sterile water to a total volume of 25 μl, 37 ℃ water bath for 5 hours, the digested product was extracted with phenol-chloroform, precipitated with absolute ethanol (2.5 times the volume) and washed with T 4 DNA ligase connects the PCR product with the expression vector pGEX-5x-1 to form a recombinant ...
Embodiment 3
[0077] Example 3: Obtaining recombinant Escherichia coli BL21 (rBmKCTL / pGEX)
[0078] The ligation product in Example 2 was transformed into Escherichia coli BL21. Inoculate Escherichia coli BL21 in 3ml of LB liquid medium (tryptone 10 g / L, yeast extract 5 g / L, sodium chloride 10 g / L), and culture on a shaker at 37°C until OD 600 When it reaches 0.4, centrifuge at 12000 rpm for 5 minutes, remove the supernatant, add ice-precooled 0.1MCaCl 2 120 μl of resuspended bacteria. Add 4 μl (<20 ng) of the ligation product, place in an ice bath at 10°C for 30 minutes, heat shock at 42°C for 90 seconds, and quickly transfer to an ice bath at 10°C for 1-2 minutes. Add 1ml of SOC (tryptone 10g / L, yeast extract 5g / L, sodium chloride 10g / L, potassium chloride 2.5mmol / L, magnesium chloride 10mmol / L, glucose 20mmol / L) solution, shake at 37°C Incubate on the bed for 1 hour, centrifuge at 1200 rpm for 5 minutes, remove the supernatant, add 100 μl of SOC solution to resuspend the bacteria, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com