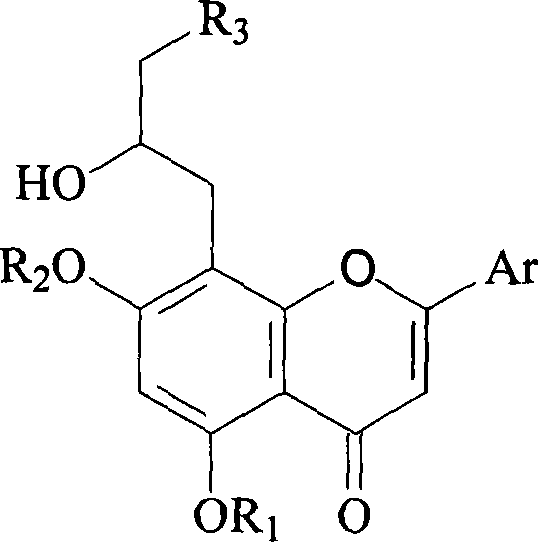
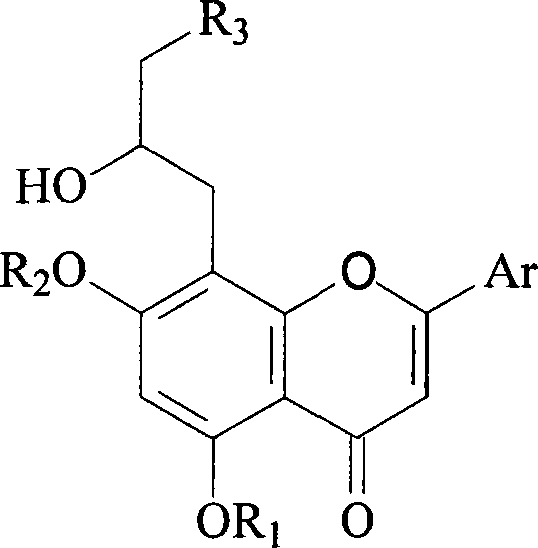
Preparation method and usage for nitrogen-containing flavone derivatives
A technology of derivatives and flavonoids, applied in the field of organic compound synthesis, can solve problems such as inefficiency, and achieve the effects of low production cost, mild reaction conditions and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
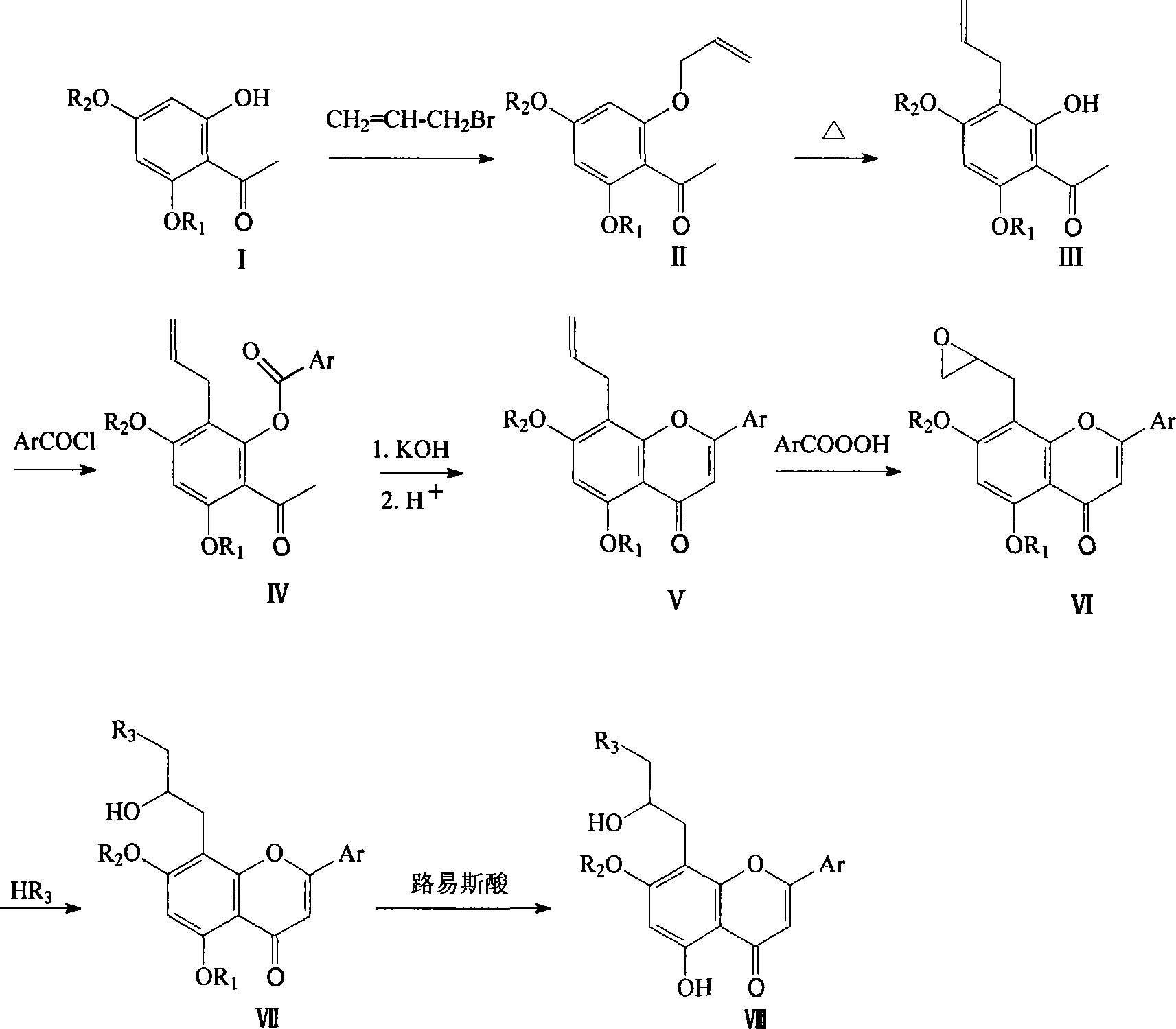
[0023] Example 1: 2-hydroxy-4,6-dimethoxy-3-allyl acetophenone (compound III)
[0024] 3.9g (20.0mmol) of compound I, 4g (30.0mmol) of anhydrous potassium carbonate and 30mL of anhydrous acetone were put into the reaction flask, and 17.3mL (20.0mmol) of allyl bromide was added dropwise with stirring. After the addition, the temperature was raised to reflux for 4 hours. The reaction solution was cooled and filtered with suction, and the filtrate was evaporated to remove the solvent to obtain 4.5 g of compound II, which was directly used in the next reaction.
[0025] Compound II was dissolved in DMF (20mL), heated and refluxed for 6h, the reaction solution was distilled off the solvent under reduced pressure, diluted with water, extracted with dichloromethane (40mL×3), washed with saturated sodium chloride solution (40mL×3), anhydrous Na2SO4 dried. After filtration, the filtrate was recovered under reduced pressure, and the crude product was subjected to column chromatography...
Embodiment 2
[0026] Embodiment 2: 2-chlorobenzoic acid-(2-acetyl-3,5-dimethoxy-6-allyl)phenol ester (compound IV 1 )
[0027] 2.4 g (10.0 mmol) of compound III was dissolved in 16 mL of dry pyridine, and 1.9 g (11.0 mmol) of o-chlorobenzoyl chloride was slowly added dropwise under cooling in an ice-water bath. After the addition was complete, stir overnight at room temperature. The dilute HCl solution containing crushed ice was poured into the above reaction solution and stirred evenly, a white solid was precipitated, filtered with suction, and the solid was dried under reduced pressure, with a yield of 86%.
Embodiment 3
[0028] Embodiment 3: 4-trifluoromethylbenzoic acid-(2-acetyl-3,5-dimethoxy-6-allyl)phenol ester (compound IV 2 )
[0029] Compound III 1.5g (6.4mmol) and p-trifluoromethylbenzoic acid 1.4g (6.9mmol) were dissolved in dry pyridine 16mL, and SOCl was slowly added dropwise at room temperature 2 (0.4 mL). After addition, stir at room temperature for 12h. The dilute HCl solution containing crushed ice was poured into the above reaction solution and stirred evenly, a white solid was precipitated, filtered with suction, and the solid was dried under reduced pressure, with a yield of 86%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com