Method for separating preparation of corn-flower pigment-3-amylaceum glycosides from red bayberry
A technology of cyanidin and glucoside is applied in the field of deep processing of bayberry, separation and preparation of cyanidin-3-glucoside from bayberry, can solve the problem that C3G is not reported in the literature, etc., and achieves high sample recovery rate and separation. good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
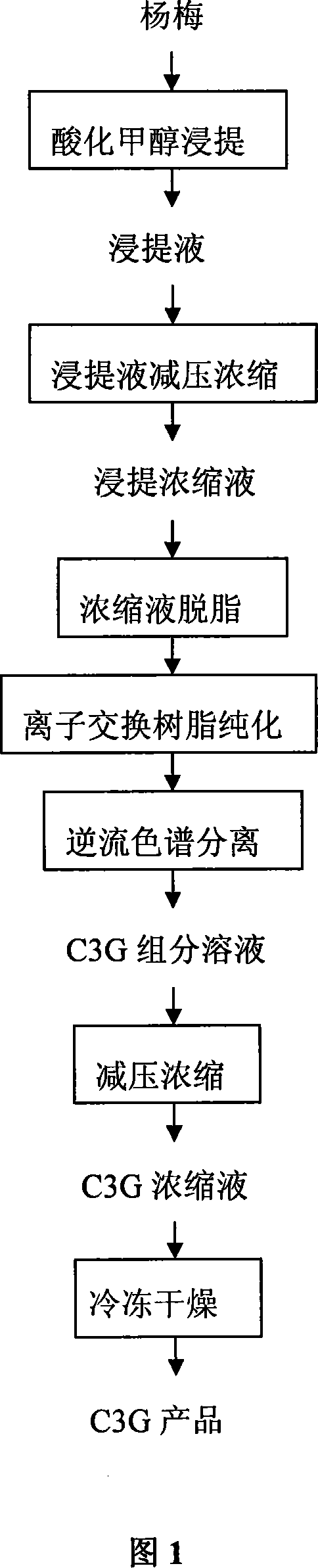
[0024] Process flow of the present invention is with reference to Fig. 1:
[0025] 1. Use methanol containing 5% formic acid or ethanol containing 5% formic acid as the solvent, the volume ratio of the extraction solvent to the bayberry is 5-10:1, and extract in the dark at room temperature for 24 hours and the number of extractions 2 to 3 times.
[0026] 2. Concentrate the extraction solution in vacuum until the solid content accounts for more than 60% of the concentrated anthocyanin solution.
[0027] 3. Add 1 to 2 times the amount of water according to the volume of the concentrated liquid to the concentrated liquid of bayberry anthocyanins obtained in step 2, and then extract twice with ethyl acetate to degrease, and the water phase is adsorbed by a cation exchange resin of the type DIAION HP2MGL. Then use acidified methanol for desorption treatment to obtain the preliminarily purified anthocyanin eluate.
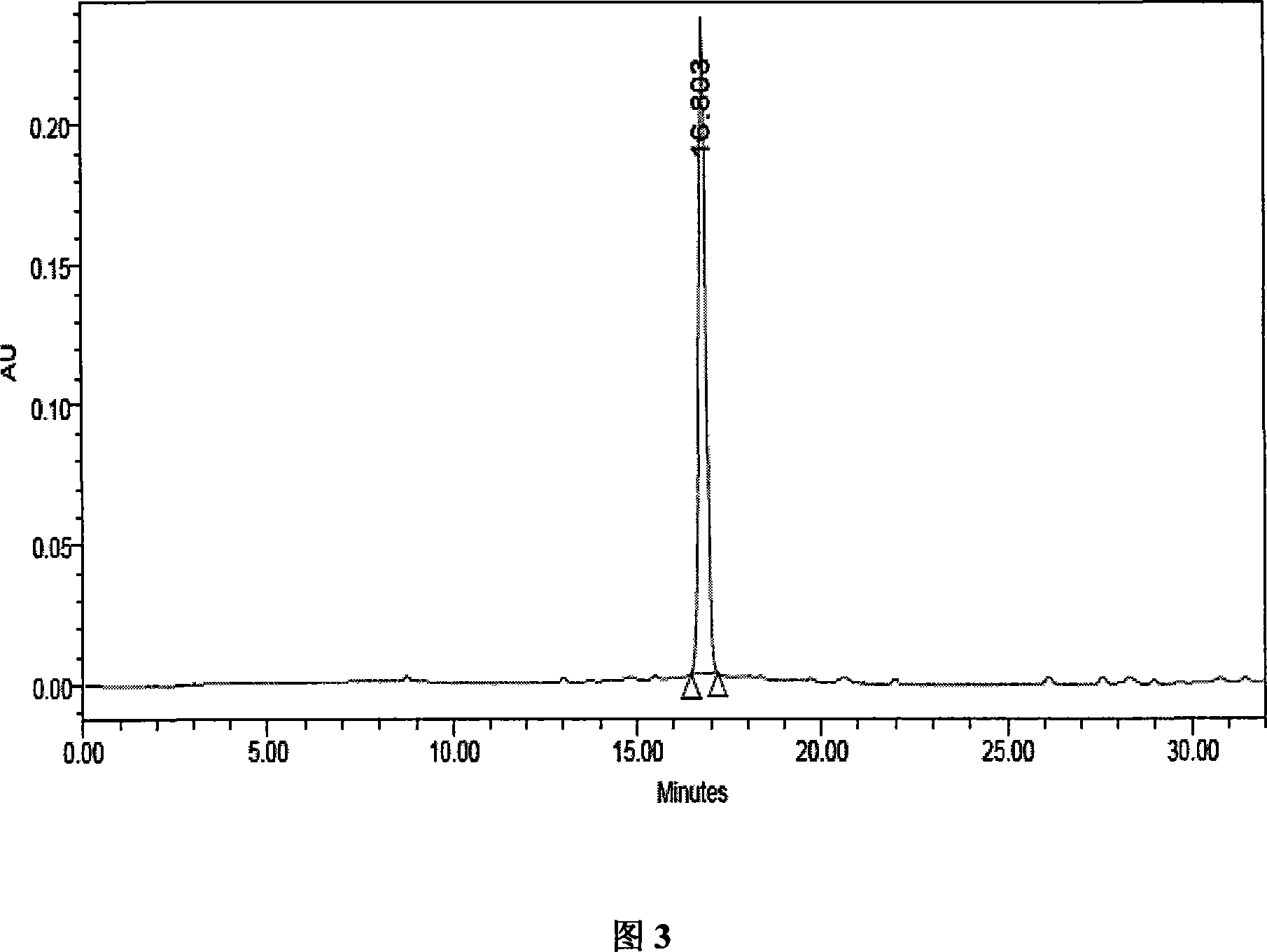
[0028] 4. Vacuum-concentrate the anthocyanin eluate obtained in ...
Embodiment 2
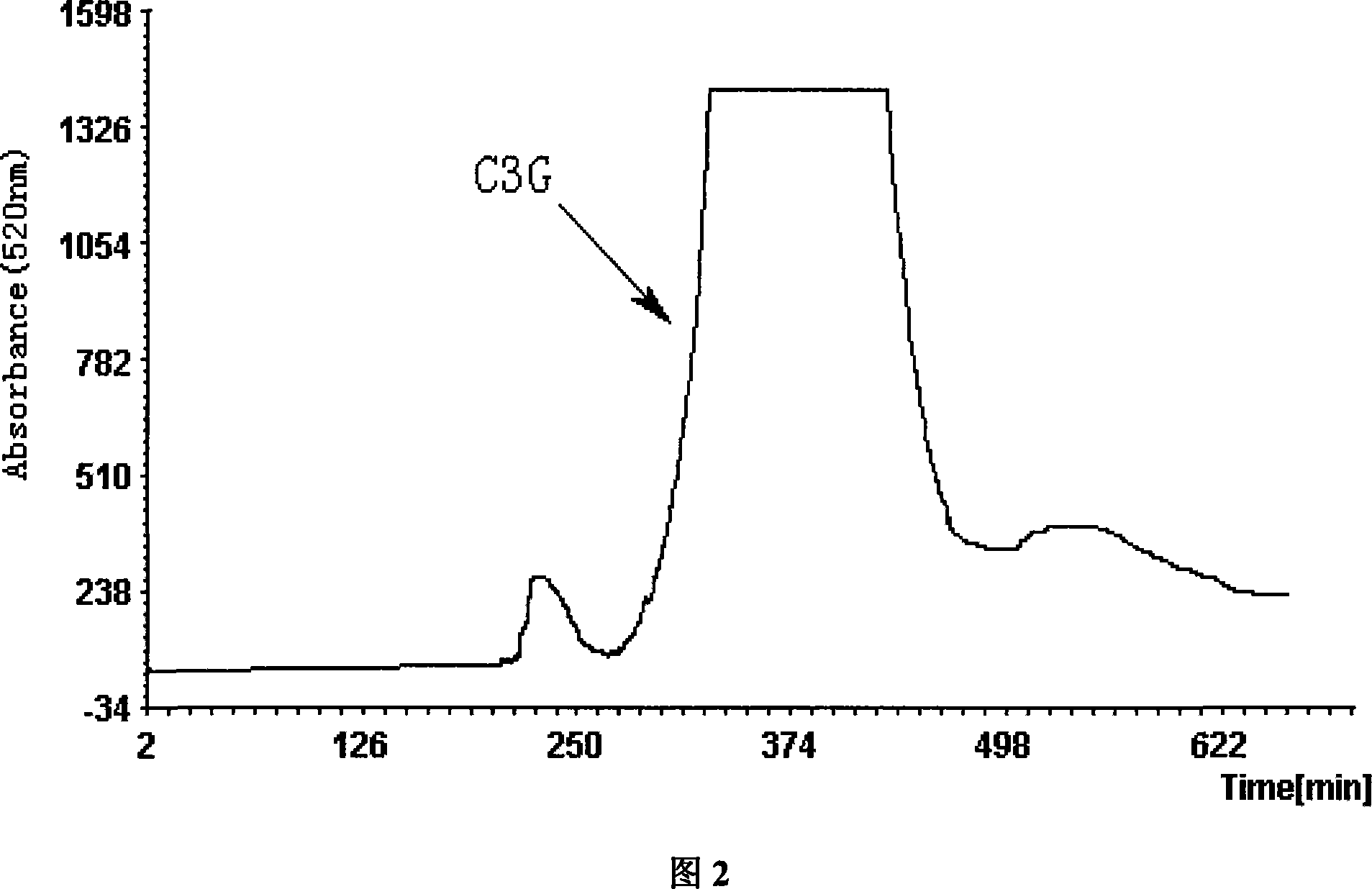
[0037] The solvent system of this embodiment adopts TBME: acetonitrile: n-butanol: TFA aqueous solution=2:1:4:8, the concentration of TFA aqueous solution is 1%, the countercurrent chromatograph is HSCCC-D1000 high-speed countercurrent chromatograph, myrica rubra anthocyanin extract Among them, C3G accounts for about 53%.
[0038] Measure 400ml of TBME, 200ml of acetonitrile, 800ml of n-butanol, 16ml of TFA and 1600ml of water, put them in a 3000ml separatory funnel, shake them well, and put the upper and lower phases into reagent bottles respectively after standing for stratification. The upper phase was injected into the chromatographic column of high-speed countercurrent chromatography at a flow rate of 40ml / min. 2.0g of C3G crude extract was dissolved in 50ml of the lower phase to prepare a countercurrent chromatography sample solution. Turn on the countercurrent chromatograph to 800 rpm, and then input the sample solution at a flow rate of 1.0ml / min. Input the flow rate ...
Embodiment 3
[0040] The solvent system of this embodiment adopts TBME: acetonitrile: n-butanol: TFA aqueous solution = 2: 1: 4: 10, the concentration of TFA aqueous solution is 0.1%, the countercurrent chromatograph is SRCCC-D3500 low-speed countercurrent chromatograph, myrica rubra anthocyanin extract Among them, C3G accounts for about 53%.
[0041] Measure 1600ml of TBME, 800ml of acetonitrile, 3200ml of n-butanol, 8ml of TFA and 8000ml of water, put them in a 15000ml glass bottle, shake them well, and put the upper and lower phases into the reagent bottles respectively after standing for stratification. The upper phase was injected into the chromatographic column of low-speed countercurrent chromatography at a flow rate of 60ml / min. Weigh 6.5g of C3G crude extract and dissolve it in 180ml lower phase to prepare a countercurrent chromatography sample solution, turn on the low-speed countercurrent chromatograph to 80 rpm, and then input the sample solution at a flow rate of 2.0ml / min. In...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com