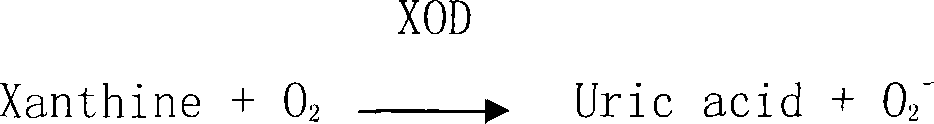Pharmaceutical preparation of biflavone compound for anti gout
A pharmaceutical preparation, biflavone technology, applied in the field of anti-gout bisflavonoid pharmaceutical preparations, can solve the problems of poor water solubility or fat solubility, limited curative effect, and low dispersion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Example 1 Experiments of Biflavone Compounds and Compositions Inhibiting Xanthine Oxidase in Vitro
[0016] 1.1 Sample materials, reagents
[0017] Biflavone, Robustaflavone, Robustaflavone-4'--methyl ether and other bisflavone compounds are monomers isolated from medicinal plants of the genus Selaginella in the laboratory of Hubei University of Traditional Chinese Medicine The compound is confirmed by structural identification, and the content is more than 97% calculated by the normalization method. The biflavonoid compound composition is a medicinal plant extract mainly containing various biflavonoid compounds extracted from medicinal plants, and its total flavonoid content is 35% to 45%. See Table 1 for the ratios of robusta flavone and robusta flavone-4'-methyl ether.
[0018] make up
combination
Suohua fir pair
Relative content of flavonoids%
Loposta Biflavone Phase
For content%
Roposta Biflavone-
4'-...
Embodiment 2
[0038] Example 2, the experiment of biflavone compound and its composition inhibiting lipoxygenase in vitro
[0039] 2.1 Materials, instruments and reagents
[0040] The composition of biflavone compound and several biflavone compounds is the same as in Example 1.
[0041] Instruments and Reagents DU640 Spectrophotometer (Beckman Inc. USA) with kinetic / time software. Lipoxygenase LOX (Sigma, lot118F03422), linoleic acid (Sigma, lot015K1200); quercetin (10081-9906); dimethyl sulfoxide and Tween-20 (Tween-20 Amresco), and other reagents were of analytical grade .
[0042] 2.2 Method
[0043] 2.2.1 Test solution preparation: 3.3 mmol / L substrate solution: Take 157.2 microliters of linoleic acid, 157.2 microliters of Tween-20 (Tween-20), 10 milliliters of water, and add 0.5 milliliters of 1 mol / liter NaOH Make the solution clear, add water to 25 ml, mix well to obtain substrate stock solution. The substrate stock solution was diluted with 0.2 mol / L phosphate buffer solution t...
Embodiment 3
[0055] Embodiment 3, the pharmacodynamic experiment of anti-hyperuricemia in vivo
[0056] 3.1 Experimental materials
[0057] Male Kunming mice, weighing (24±2) grams, were purchased from the Experimental Animal Center of Hubei Province. The 80% ethanol extract of a certain medicinal plant, in which the total flavonoid content is greater than 35%, and the biflavonoid compounds contained in it are Biflavone, Robosta Biflavone, Robosta Biflavone-4'--Methyl Ether The ratio is 75:15:5.
[0058] 2. Experimental method
[0059] 40 male mice were randomly divided into 4 groups, 10 in each group, administered once a day i.g (gavage), for 4 consecutive days, wherein the normal saline group and hyperuricemia model group were dosed i.g. 100 ml / kg every day. Normal saline, hyperuricemia model group received ip (intraperitoneal injection) of oxonic acid potassium salt 2 hours before blood collection, and ip oxonic acid potassium salt in the administration group one hour before the last...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com