Anethol trithione drop pills, and preparation method
A technology of sulfur dripping pills and anisitrazine, which is applied in the field of anisitrazine dripping pills and preparation thereof, can solve the problems of difficulty in swallowing, low bioavailability, long disintegration time, etc., and is beneficial to labor protection and environmental protection, High bioavailability and the effect of reducing dust pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
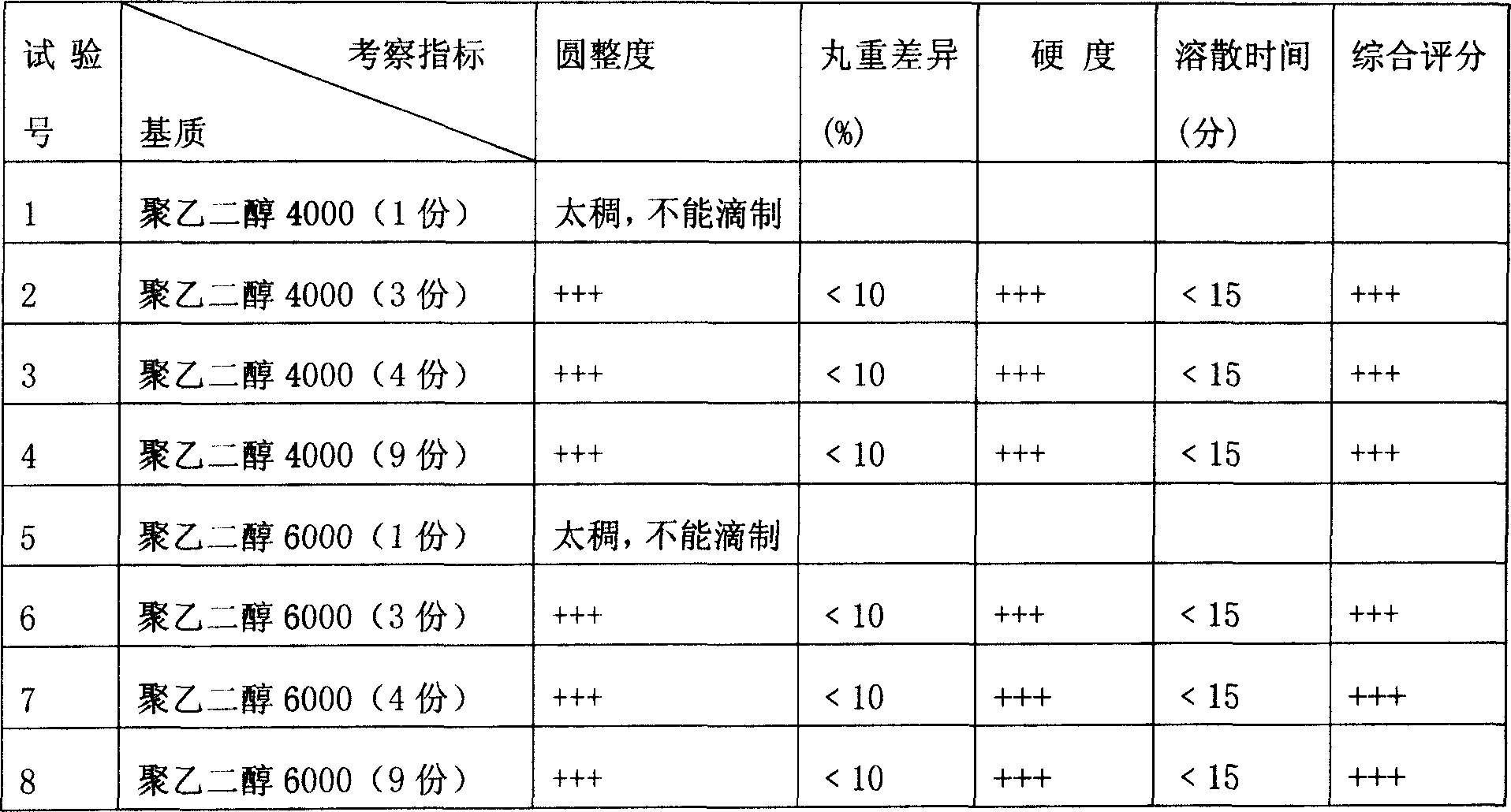
[0025] Example 1: In this example, through the formulation of anetellithin and a single matrix, the operation is carried out according to the preparation method in the [specific implementation method]. Hardness, dissolving time, etc. are used as indicators to observe the influence of the weight ratio of the drug to the single matrix on the product involved in the present invention. The test results are shown in Table 1.
[0026] Table 1 Tests of drug and single matrix formulation (all drugs are 1 part)
[0027]
[0028] Note: 1. The coolant is simethicone oil, and the cooling temperature is 8-5°C; the heat preservation temperature of the drug material and the dripper is 85-90°C; the dripping speed is 30-50 grains / minute.
[0029] 2. The above results show that the indicators of No. 2, 3, 4, 6, 7, and 8 tests are better, that is, the drug can be dripped smoothly when the ratio of drug to matrix is 1:3-1:9. However, in consideration of factors such as dosage, the ...
Embodiment 2
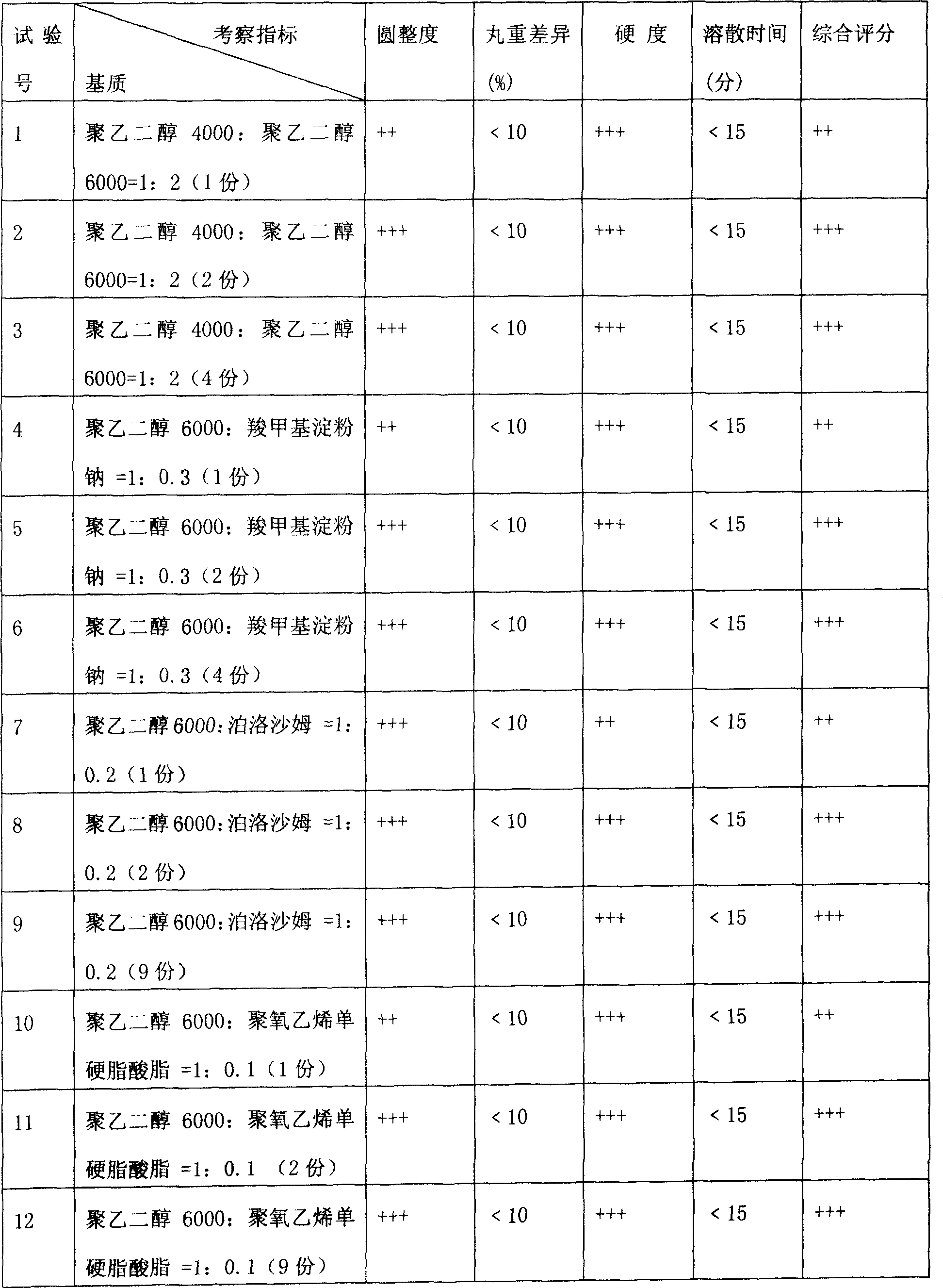
[0030] Example 2: In this example, through the formula of anetellithin and mixed matrix, operate according to the preparation method in [specific implementation mode], the coolant is simethicone oil, drop pills, and select roundness, pill weight difference, Hardness, dissolving time etc. are investigation indexes, observe the influence of the weight ratio of medicine and mixed matrix on the product involved in the present invention, test result is shown in Table 2.
[0031] Table 2 Drug and mixed matrix formulation test (1 part of drug)
[0032]
[0033] Note: 1. The coolant is simethicone oil, and the cooling temperature is 8-5°C; the heat preservation temperature of the drug material and the dripper is 85-90°C; the dripping speed is 30-50 grains / min.
[0034] 2. The above results show that the indicators of No. 2, 3, 5, 6, 8, 9, 11, and 12 tests are all good, that is, when the ratio of drug to matrix is 1:1-1:9, it can be dripped smoothly. However, in consi...
Embodiment 3
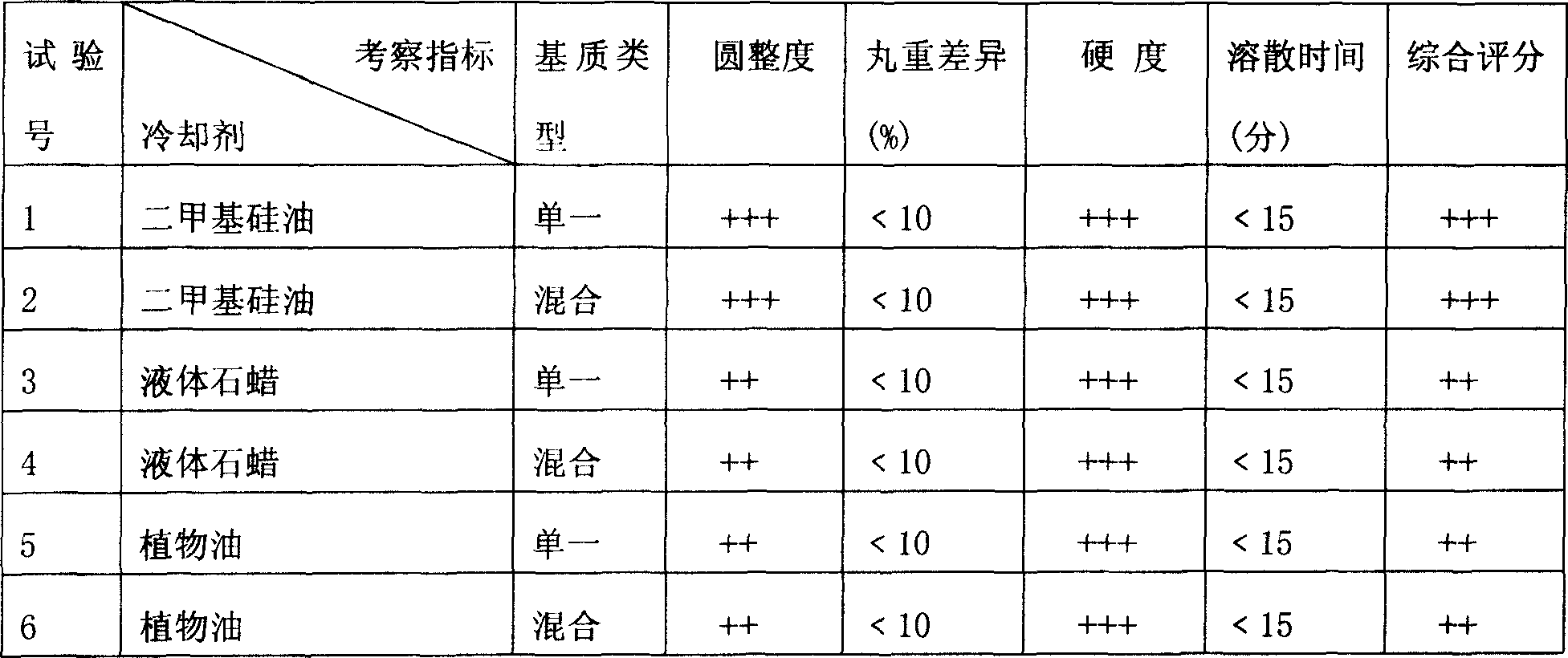
[0035] Embodiment 3: In this embodiment, different coolants are selected, and the coolant is simethicone, liquid paraffin, vegetable oil, and polyethylene glycol is selected as a single matrix 6000 , the mixed matrix chooses polyethylene glycol 6000 : the formula of poloxamer=1:0.2, operate according to the preparation method in [the specific embodiment], drip dripping pill, select roundness, pill weight difference, hardness, dissolving time etc. as investigation index, observe different The impact of coolant on the products involved in the present invention, the test results are shown in Table 3.
[0036] Table 3 Experiments using different coolants (drug: matrix = 1:2)
[0037]
[0038] Note: 1. The cooling temperature is 8-5°C; the heat preservation and dripper temperature is 85-90°C; the dripping speed is 30-50 capsules / minute.
[0039] 2. The above results show that the indicators of No. 1 and No. 2 tests are better, that is, when the above-mentioned diff...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com