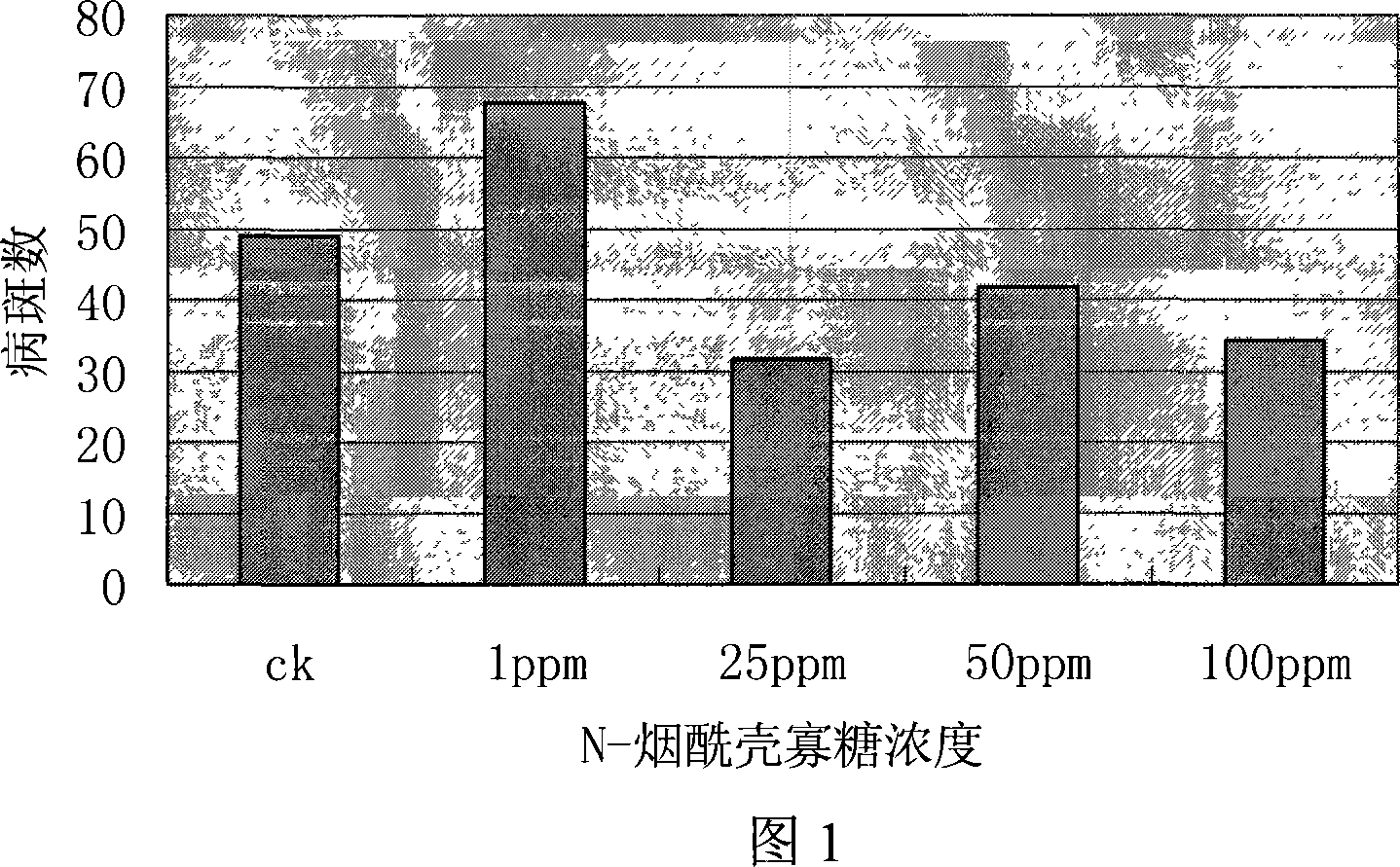
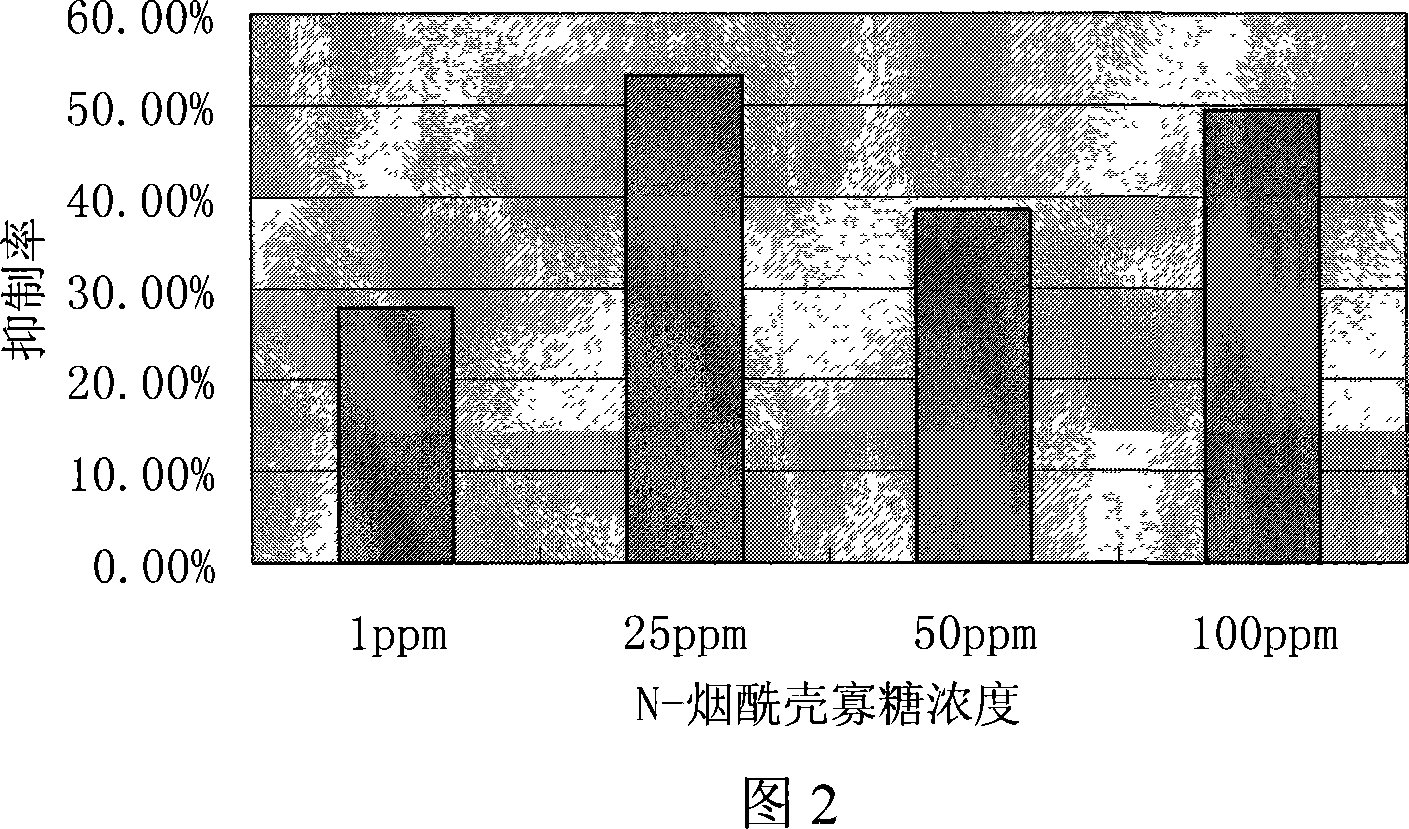
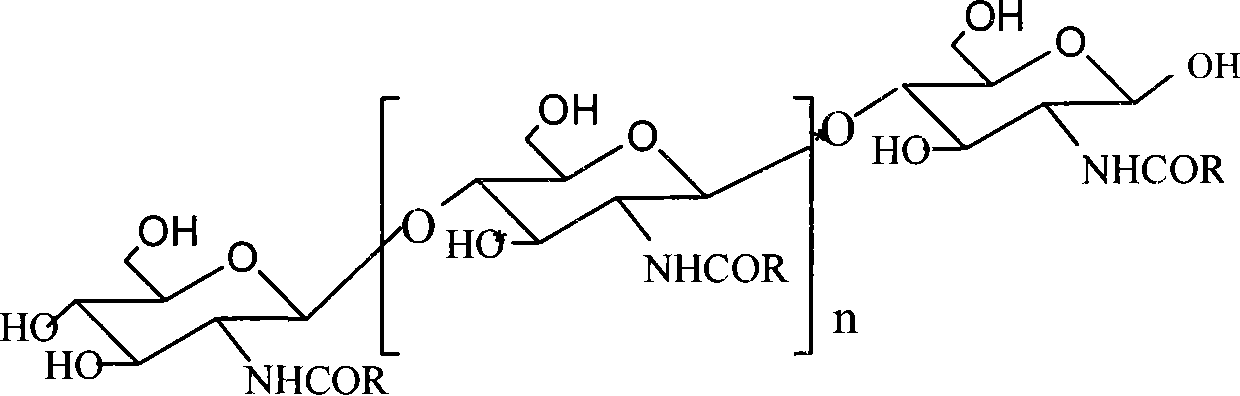
N-nicotinoyl chitosan oligosaccharide and preparation method thereof
A kind of nicotinyl chitosan oligosaccharide and nicotinyl oligosaccharide technology, applied in the field of chitosan oligosaccharide
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: the preparation of N-nicotinoyl chitosan oligosaccharide (ammonolysis method):
[0028] Take 1.23g of nicotinic acid and dissolve it in N,N-dimethylformamide (DMF) (the solvent can also be pyridine, dimethyl sulfoxide, dichloromethane, etc.), add 2.7g of p-nitrophenol, stir, and add 0.1 g dimethylaminopyridine, add dropwise a solution of 2.06g dicyclohexylcarbodiimide dissolved in 5mL DMF, react under heating conditions at 60-70°C for 2-6h after dropping, filter, pour the filtrate into ice water, and precipitate White crystals, filtered, and recrystallized with absolute ethanol to obtain 1.0 g of p-nitrophenyl nicotinate,
[0029] Nicotinic acid p-nitrophenyl ester can also be prepared according to the following method:
[0030] Add an appropriate amount of anhydrous acetone to a dry reaction flask containing 0.8 g of nicotinic acid chloride under ice bath, stir vigorously, add 10 mL of anhydrous triethylamine and 1.0 g of p-nitrophenol in 20 mL of aceton...
Embodiment 2
[0034] Embodiment 2: the preparation of N-nicotinoyl chitosan oligosaccharide (acyl chloride method)
[0035] The preparation of nicotinoyl chloride: 10mLSOCl 2 Put it into a reaction flask equipped with an HCl gas absorption device, add 1.67g of nicotinic acid to it under the protection of nitrogen at -10~5°C, stir, drop a few drops of anhydrous DMF, and slowly raise the temperature to 90°C in an oil bath, After reflux for 3 h, excess SOCl was distilled off under reduced pressure 2 , to obtain nicotinoyl chloride as a yellow solid with a yield of 80%, which can be reacted without separation.
[0036] Take 3.0g of nicotinyl chloride prepared from nicotinic acid and dissolve it in 50mL of DMF; 2 CO 3 Dissolve 2g in 20mL of water, add 1.6g of chitosan oligosaccharide (COS) and stir to make it fully dissolved, slowly add its acid chloride solution dropwise to the chitosan oligosaccharide (COS) solution under nitrogen protection and low temperature (-10°C ~ 5°C) In, after dri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com