Method of preparing hydrogenated terpinene maleic anhydride glycidyl ester type epoxy resin
A technology of glycidyl ester and hydrogenated terpene, which is applied in the field of manufacturing hydrogenated terpene maleic anhydride glycidyl ester epoxy resin, can solve the problems of thermal stability, poor UV resistance and weather resistance, dark color and the like, and achieves a simple manufacturing process. , Simplify the synthesis process steps, the effect of the reaction is good
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
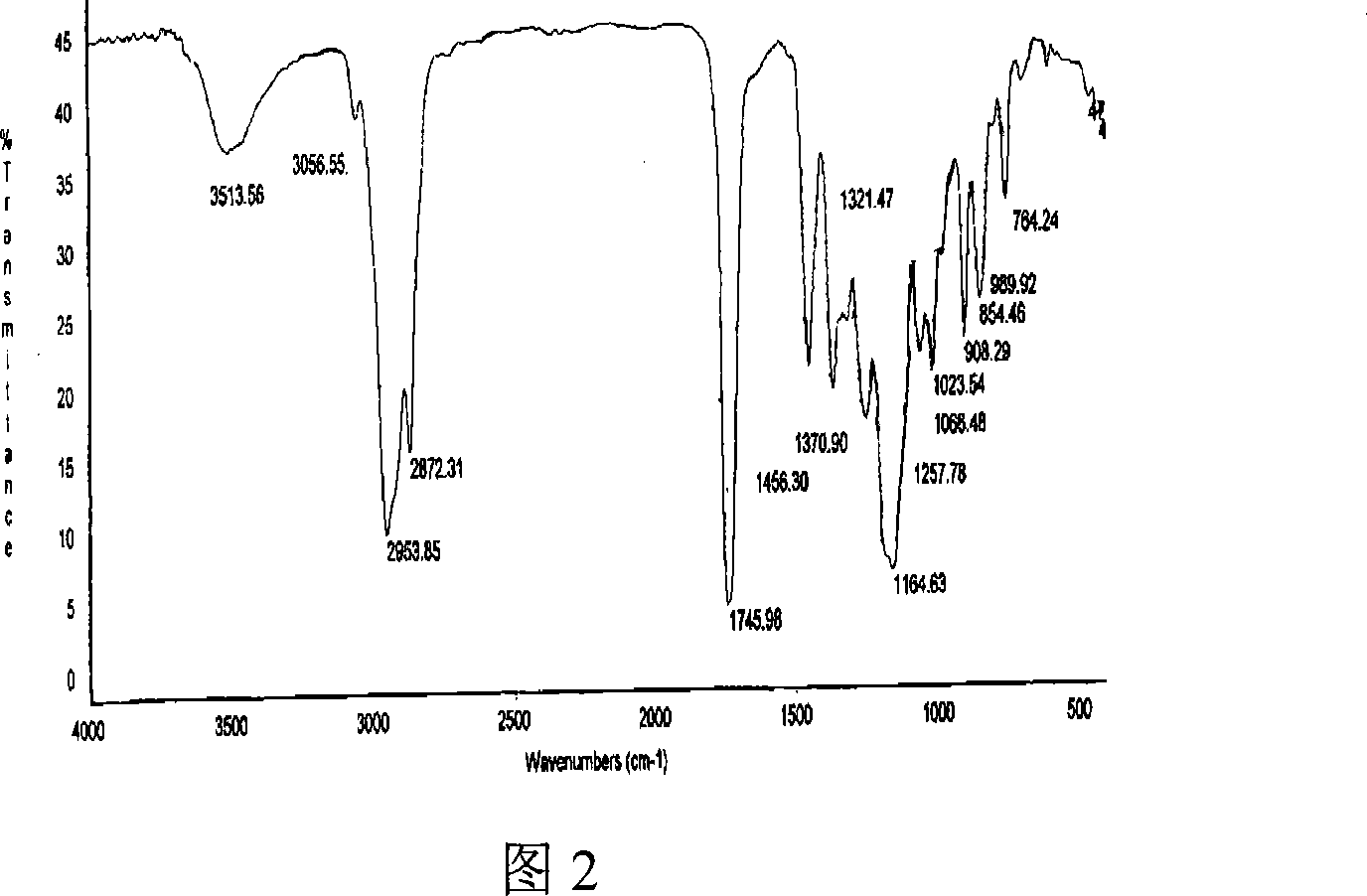
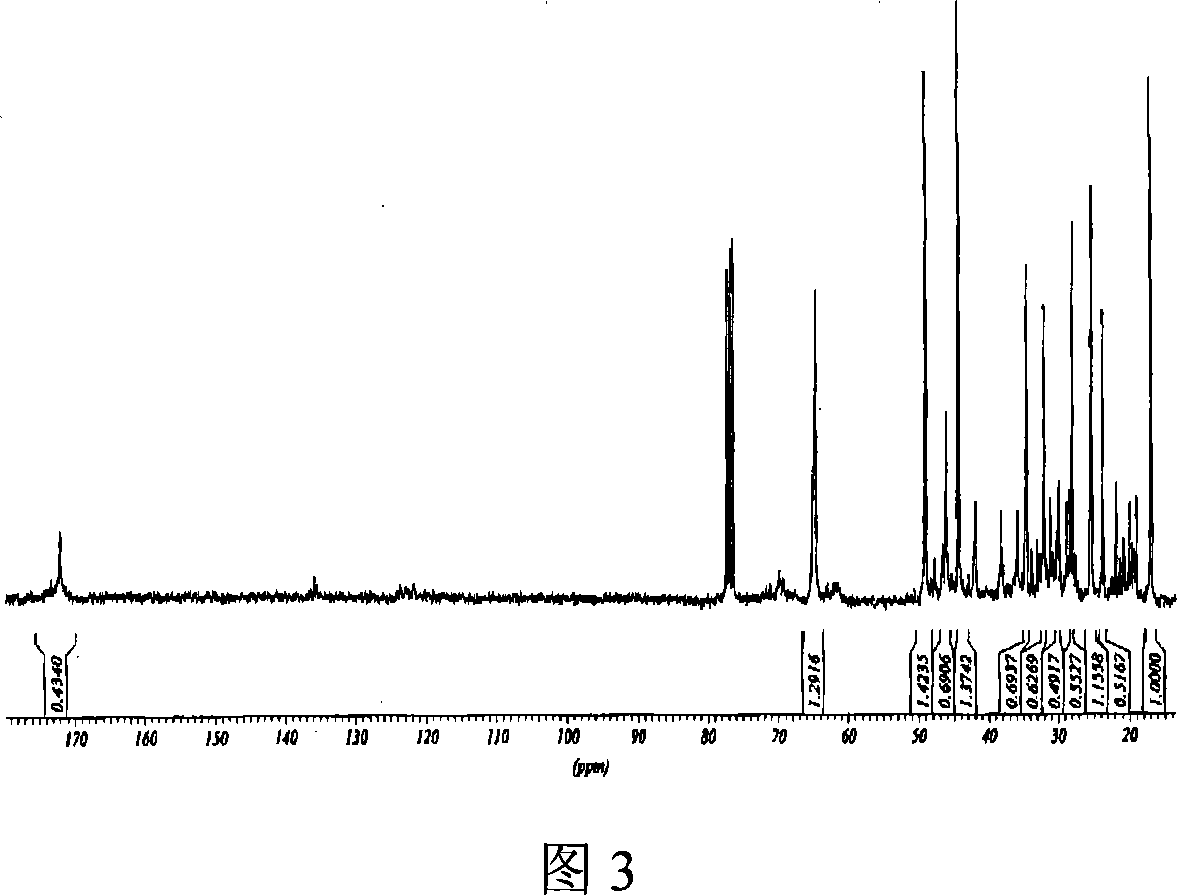
Image
Examples
Embodiment 1
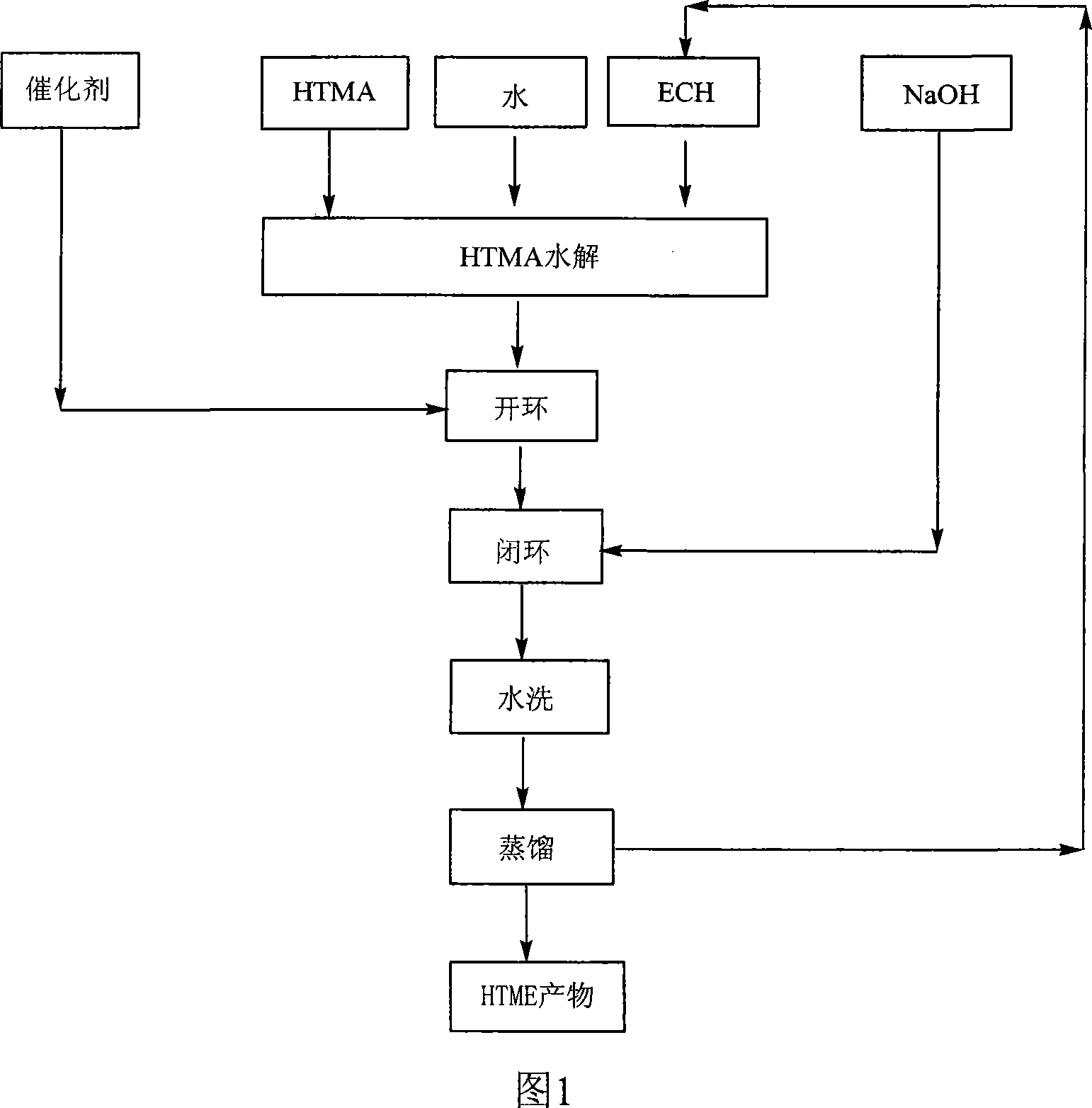
[0021] In a four-necked bottle equipped with a thermometer, a stirrer, and a condenser, add 66.3 parts by mass of HTMA, 7.5 parts by mass of H 2 O and 260.4 parts by mass of ECH are mixed and heated to an azeotropic reflux state for 2.5 hours, then 1 part by mass of catalyst benzyltriethylammonium chloride is added, and reflux is continued for about 2 hours; then the temperature is lowered to about 50°C, and the Within 2 hours, a total of 21.3 parts by mass of solid NaOH was added in batches to close the ring of the reactant, and then the temperature was raised to about 70° C. for 2 hours. Wash with hot water until neutral, remove NaCl, and distill off excess ECH under reduced pressure to obtain the product as a light yellow transparent liquid with a yield of 94.8% and an epoxy value of 0.37eq / 100g.
Embodiment 2
[0023] In a four-necked bottle equipped with a thermometer, a stirrer, and a condenser, add 83.1 parts by mass of HTMA, 9.7 parts by mass of H 2 O and 261.4 parts by mass of ECH, mixed and heated to azeotropic reflux state, maintained for 2.5 hours, then added 1 part by mass of catalyst tetrabutylammonium chloride, continued to reflux for about 3 hours; then cooled to about 50 ° C, in 2 hours A total of 27 parts by mass of solid NaOH was added in batches to close the ring of the reactant, and then the temperature was raised to about 75° C. for 2 hours. Wash with hot water until neutral, remove NaCl, and distill off excess ECH under reduced pressure to obtain the product as a light yellow transparent liquid with a yield of 92.6% and an epoxy value of 0.36eq / 100g.
Embodiment 3
[0025] In a four-necked bottle equipped with a thermometer, a stirrer, and a condenser, add 66.6 parts by mass of HTMA, 6.6 parts by mass of H 2 O and 313.8 parts by mass of ECH are mixed and heated to an azeotropic reflux state for 2.5 hours, then 1 part by mass of catalyst benzyltriethylammonium chloride is added, and reflux is continued for about 2 hours; then the temperature is lowered to about 50°C, and the Within 2 hours, a total of 21.4 parts by mass of solid NaOH was added in batches to close the ring of the reactants, and then the temperature was raised to about 70° C. for 2 hours. Wash with hot water until neutral, remove NaCl, and evaporate excess ECH under reduced pressure to obtain the product as a light yellow transparent liquid with a yield of 96.3% and an epoxy value of 0.37eq / 100g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Epoxy value | aaaaa | aaaaa |
| Epoxy value | aaaaa | aaaaa |
| Epoxy value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com