Streptococcus pneumonia autoenzyme protein and its protocaryon expression purification technique and vaccine
A Streptococcus pneumoniae, expression and purification technology, applied in the direction of bacterial antigen components, chemical instruments and methods, biochemical equipment and methods, etc., can solve the problems of ineffective colonization and low expression level of Streptococcus pneumoniae Ly-tA
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
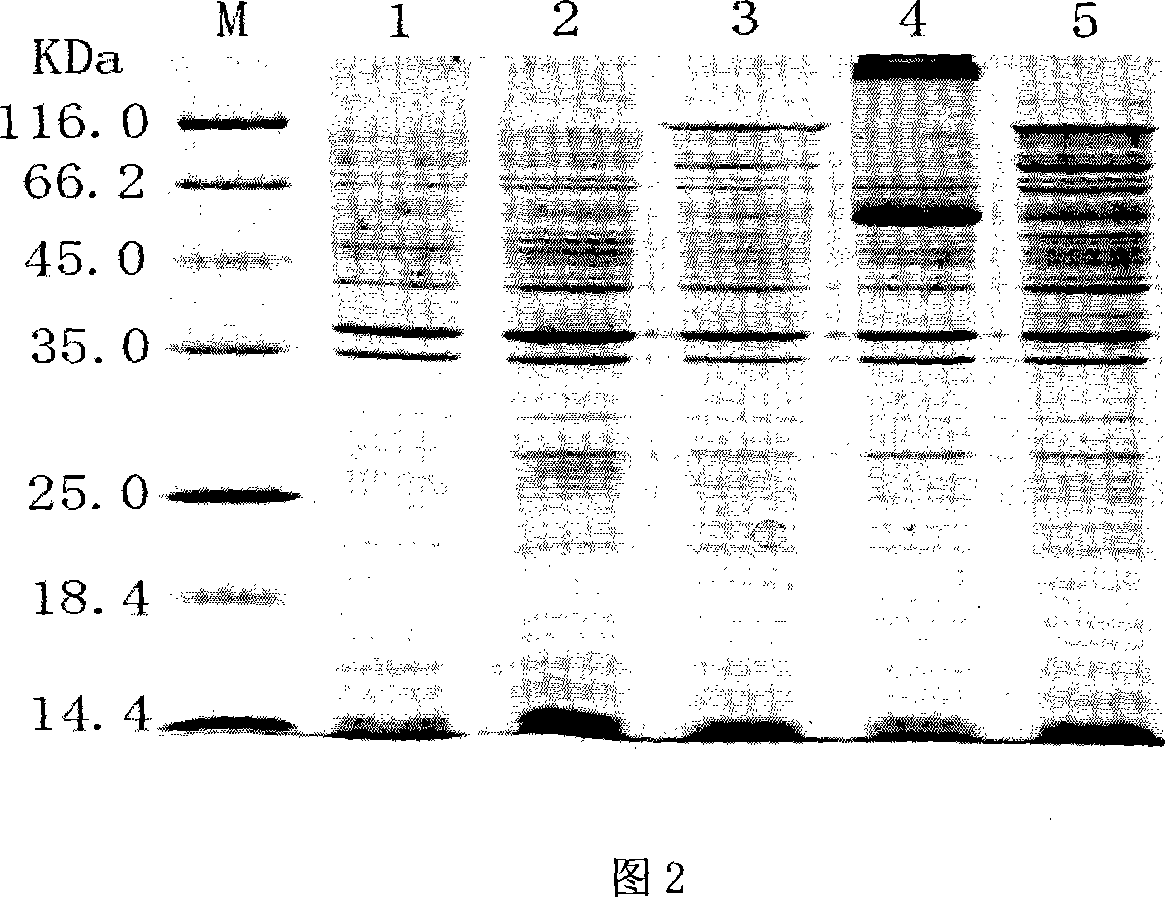
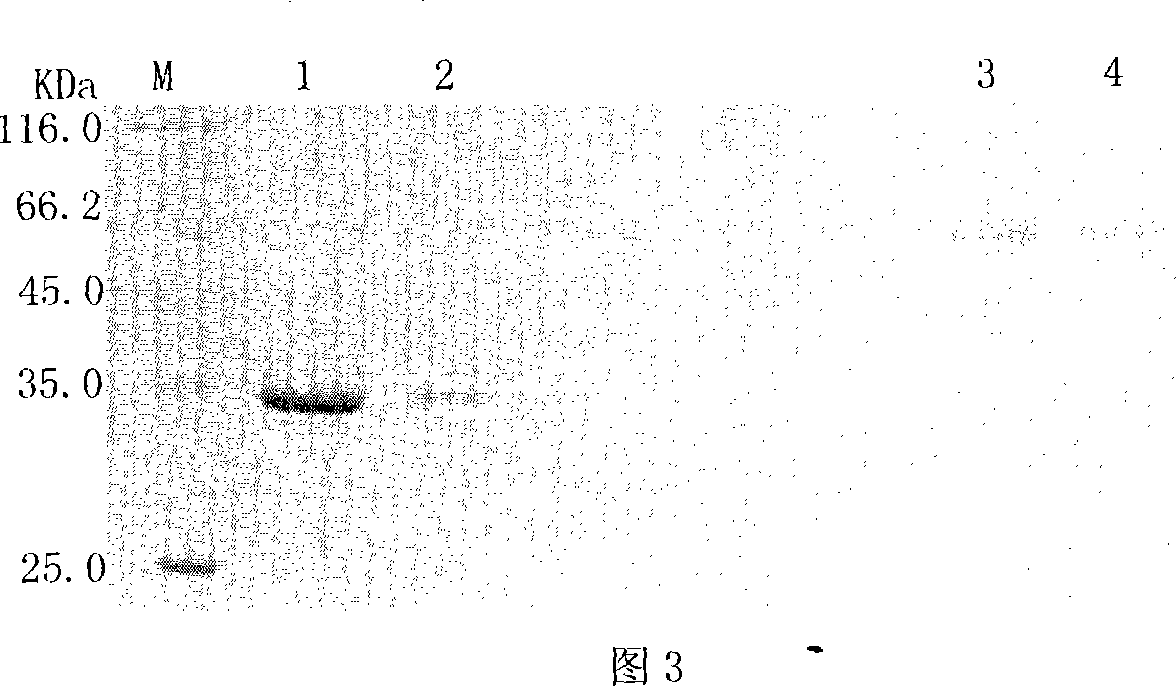
Image
Examples
Embodiment 1
[0053] Embodiment 1: Gene sequence and amino acid sequence of recombinant 23F serotype Streptococcus pneumoniae Ly-tA
[0054] The Streptococcus pneumoniae autolysozyme protein in the present invention comes from the 23F type Streptococcus pneumoniae with the strongest clinical pathogenicity. The inventors designed primers according to the autolysozyme coding gene of the international standard Streptococcus pneumoniae strain R6 strain, and obtained the coding gene by amplifying the 23F type Streptococcus pneumoniae genome. Using the analysis tools provided by NCBI ( http: / / www.ncbi.nlm.nih.gov / gorf / orfig.cgi ) to obtain its amino acid sequence.
Embodiment 2
[0055] Example 2: Identification and analysis of recombinant plasmids used in the expression of recombinant Ly-tA
[0056] Primers designed to amplify the autolysozyme gene with restriction sites.
[0057] The designed primers are:
[0058] Downstream primer: 5'-GCGCAGATCGTCAGTCAGTCAC-3'
[0059] Upstream primer: 5'-GCATGGCCTTTGCAGGGCTG-3'
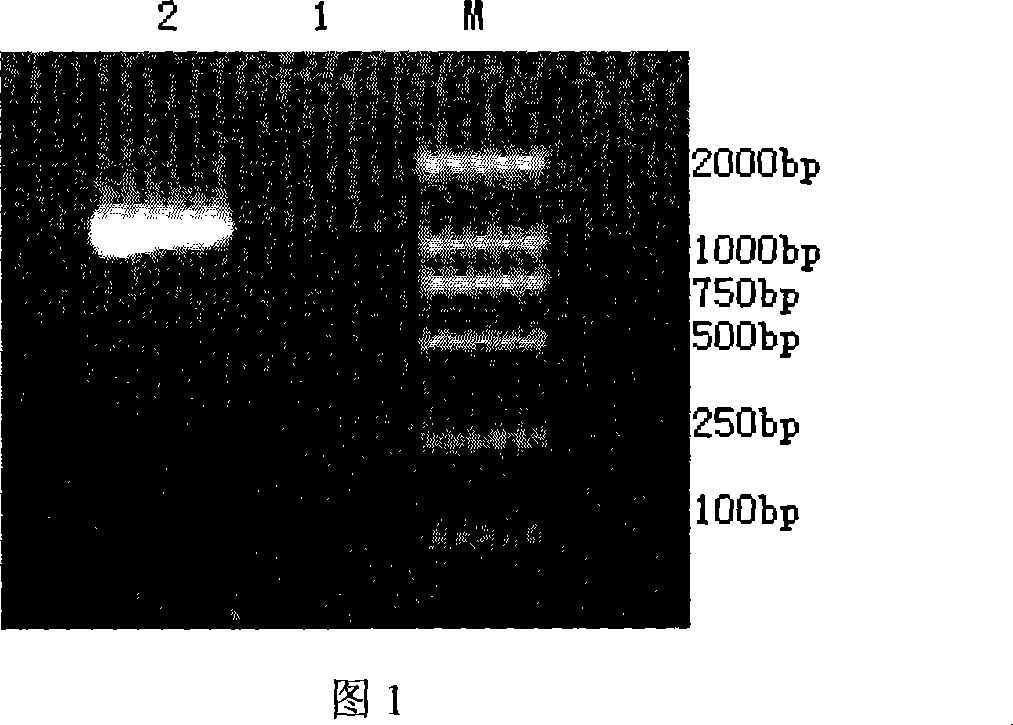
[0060] The pair of primers can be used to amplify the Ly-tA gene fragment with double restriction sites from the recombinant plasmid. The amplification reaction conditions are: 50MmKCl, 10Mm TrisCl pH 8.5, 1.5Mm MgCl in a reaction volume of 50μl 2 , 200 μmol / L dNTP, 2 μl of 10 pmol primer, 0.5U Tap DNA polymerase. On the thermal cycler of HEMA Company, the reaction was performed for 34 cycles after preheating at 95°C for 3min according to the following conditions: 94°C for 1min, 56°C for 2min, and 72°C for 2min. After the last round, extend at 72°C for 10 min. After the amplified product was purified, the DNA sequence analysis result...
Embodiment 3
[0061] Example 3: Homology analysis and protein epitope analysis of genes encoding Ly-tA of different serotypes
[0062] The LytA sequence consists of 318 amino acids. There is only one amino acid difference between the LytA amino acid sequence of 6B, 6A, 14, and 19F serotype Streptococcus pneumoniae and the 23F serotype LytA amino acid sequence. The first three are phenylalanine (Y) at 146, and the latter is Cysteine (C). Using different epitope prediction tools, it was found that there was no difference in the distribution of epitopes in the autolysin protein of different serotypes of Streptococcus pneumoniae. The serotypes are 6A, 14, and 19F three serotypes of Streptococcus pneumoniae LytA (corresponding strains are SH101, SH85, 80, respectively) gene sequence and 23F serotype LytA (Streptococcus pneumoniae strain SH137) gene sequence has only one base difference Differences, the first three are A at 746bp, and the latter is G. Compared with LytA gene sequence of sero...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com