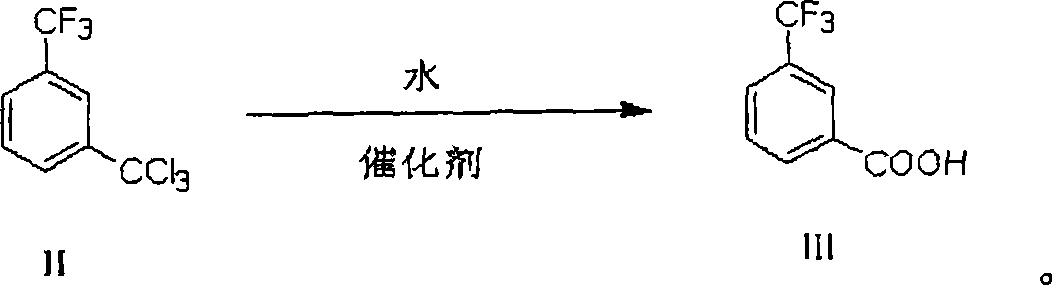
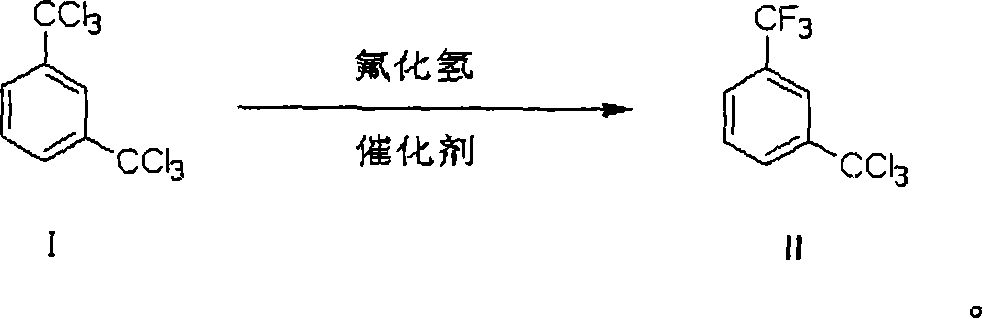
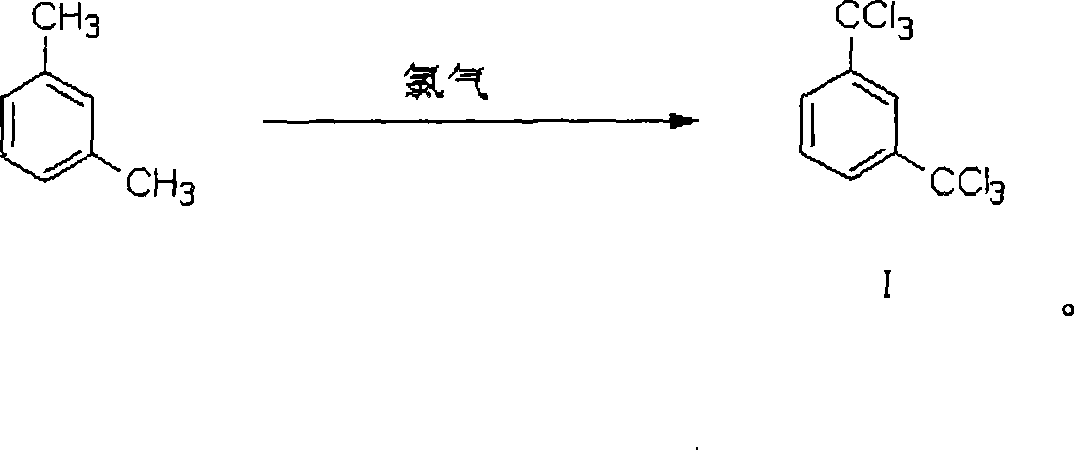
Process of preparing 3-trifluoromethyl benzoic acid
A technology of trifluoromethylbenzoic acid and trichloromethyltrifluoromethylbenzene, which is applied in the field of preparation of 3-trifluoromethylbenzoic acid, can solve problems such as unfavorable industrial production, affecting product purity, and difficulty in obtaining raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Preparation of m-Ditrichloromethylbenzene
[0065] After mixing 563 mol parts of m-ditrichloromethylbenzene and 1689 mol parts of m-xylene, the temperature in the reactor was raised to 120°C, then the ultraviolet lamp was turned on, chlorine gas was introduced, and the temperature in the reactor was maintained at 120-140°C. The reaction proceeded for 30 hours. According to the analysis by gas chromatograph, the content of m-dichloromethyltrichloromethylbenzene, an intermediate of the chlorination reaction, is less than 0.5%. Measured by GC-MS method, the reaction product is m-dichloromethylbenzene.
[0066] Degas under reduced pressure, and distill the reaction liquid under reduced pressure. Collect the fractions at 154-158℃ / 10mmHg to obtain 2068 parts of m-ditrichloromethylbenzene. The yield of the chlorination reaction is 89.3%. The purity of benzene is 97.2%.
Embodiment 2-9
[0068] Preparation of m-Ditrichloromethylbenzene
[0069] It was carried out in the same manner as in Example 1, except that the reaction temperature, raw material ratio and reaction time were as shown in Table 1. Table 1 also shows the yield and purity of m-ditrichloromethylbenzene and the content of high boilers in the resulting product.
[0070] In addition, Example 8 used 2% by weight of azobisisobutyronitrile (based on the weight of meta-xylene) as the initiator instead of UV initiation.
Embodiment 9
[0071] Example 9 uses 2% by weight of benzoyl peroxide (based on the weight of meta-xylene) as the initiator instead of UV initiation.
[0072] Implement
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com