Pyrazole or triazole compounds and their use for the manufacture of a medicament for treating somatic mutation-related diseases
A technology of somatic mutation and compound, applied in nonsense mutation related diseases and nonsense mutation related premature translation termination field, can solve the problems of no disclosure of clitocine, no report of development of clitocine analogs or derivatives, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0363] B, the preparation of the compound of the present invention
[0364] The compounds of the invention may be prepared in any manner known in the art. By way of example, compounds of the invention can be prepared according to the general methods described below for single azine ring center structures. For example, the compound wherein V of formula 1 is N can be prepared according to the method shown in the following scheme A:
[0365]
[0366] Illustration A
[0367] According to Scheme A, the unsubstituted nitrogen atom on the azole ring of compound A1 can be substituted in the cross-coupling reaction. This type of reaction can be accomplished by using substrates such as Ar 1 -X (where X is a halogen such as bromine or iodine, or a halide-like compound such as methyl sulfonate) or Ar 1 -M (wherein M is a group such as boronic acid or trialkoxysilane). Catalysts used for this reaction may include copper salts (for example: copper(II) oxide,...
Embodiment 1
[0480] Embodiment 1: the preparation of compound of the present invention
[0481] A. Preparation of pyrrole
[0482] Generally, the pyrroles of the present invention can be prepared as follows:
[0483] Preparation of 3-[1-(4-trifluoromethylphenyl)-]-1H-pyrrol-3-yl]benzoic acid sodium salt (compound 14)
[0484] Part A. To 1-(triisopropylsilyl)pyrrole-3-boronic acid (according to Alvarez, A.; Guzman, A.; Ruiz, A.; Velarde, E., J.Org.Chem.1992, 57, prepared by the method of 1653-1656) (6.12g, 22.9mmol) in anhydrous dimethoxyethane (76ml) was added methyl 4-iodobenzoate (96.61g, 25.2mmol), dichloro Bis(triphenylphosphine)palladium(II) (0.484g, 0.69mmol) and cesium fluoride (6.96g, 45.8mmol). The mixture was heated to reflux under nitrogen atmosphere for 17 hours. The reaction mixture was cooled to room temperature, diluted with water (100 mL), and extracted with ethyl acetate (4 x 25 mL). The extract was washed with water, MgSO 4 Dry and concentrate to give crude product....
Embodiment 2
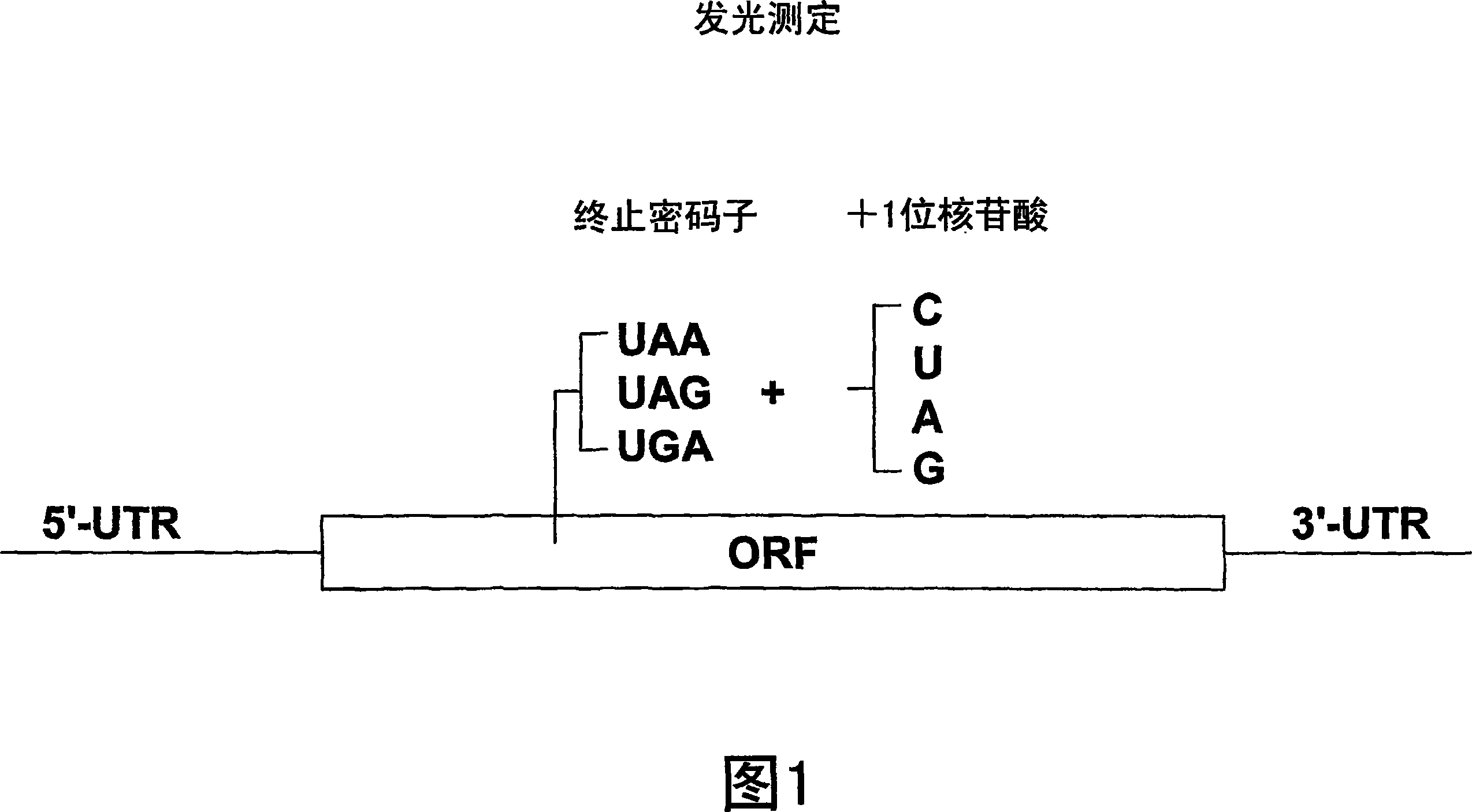
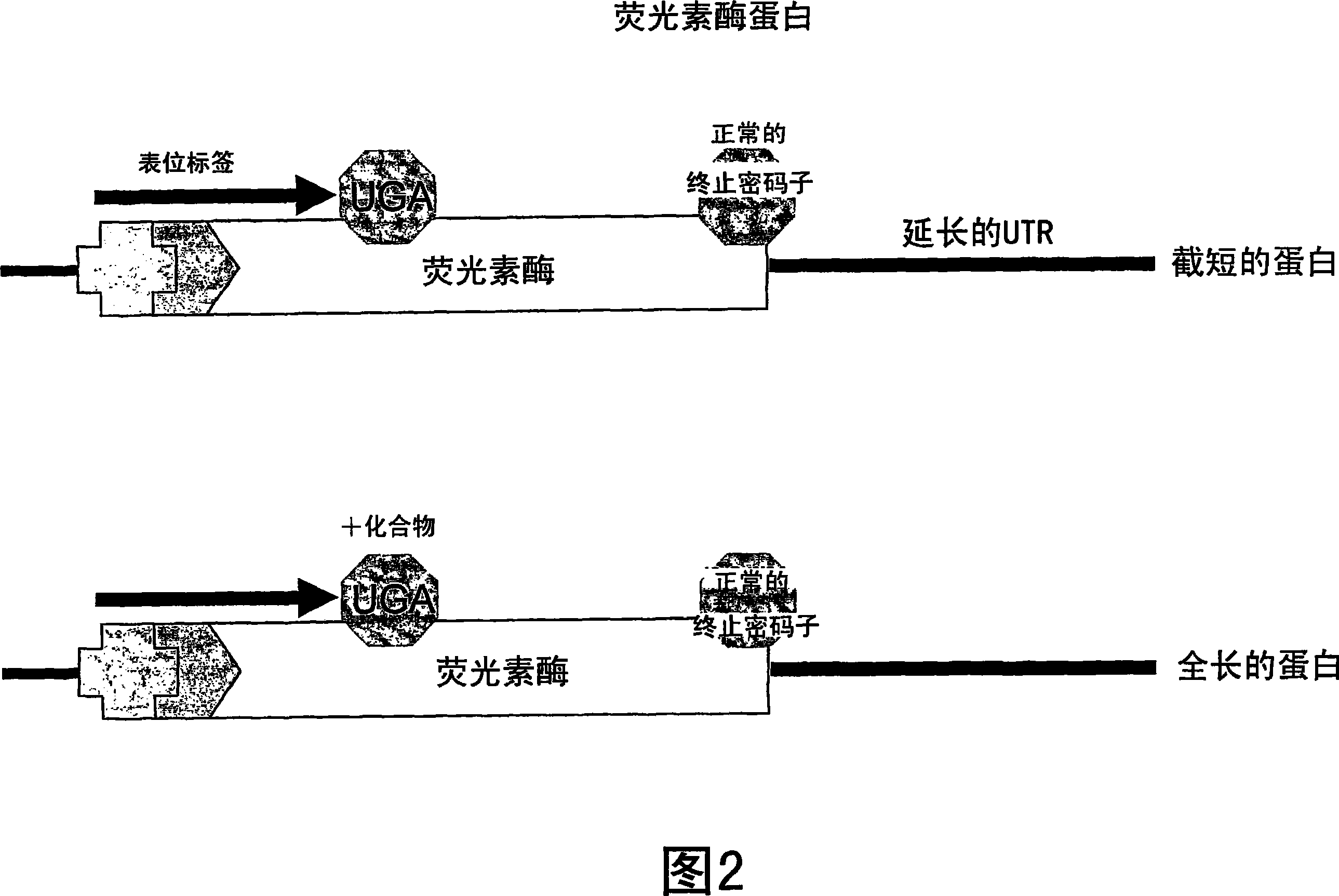
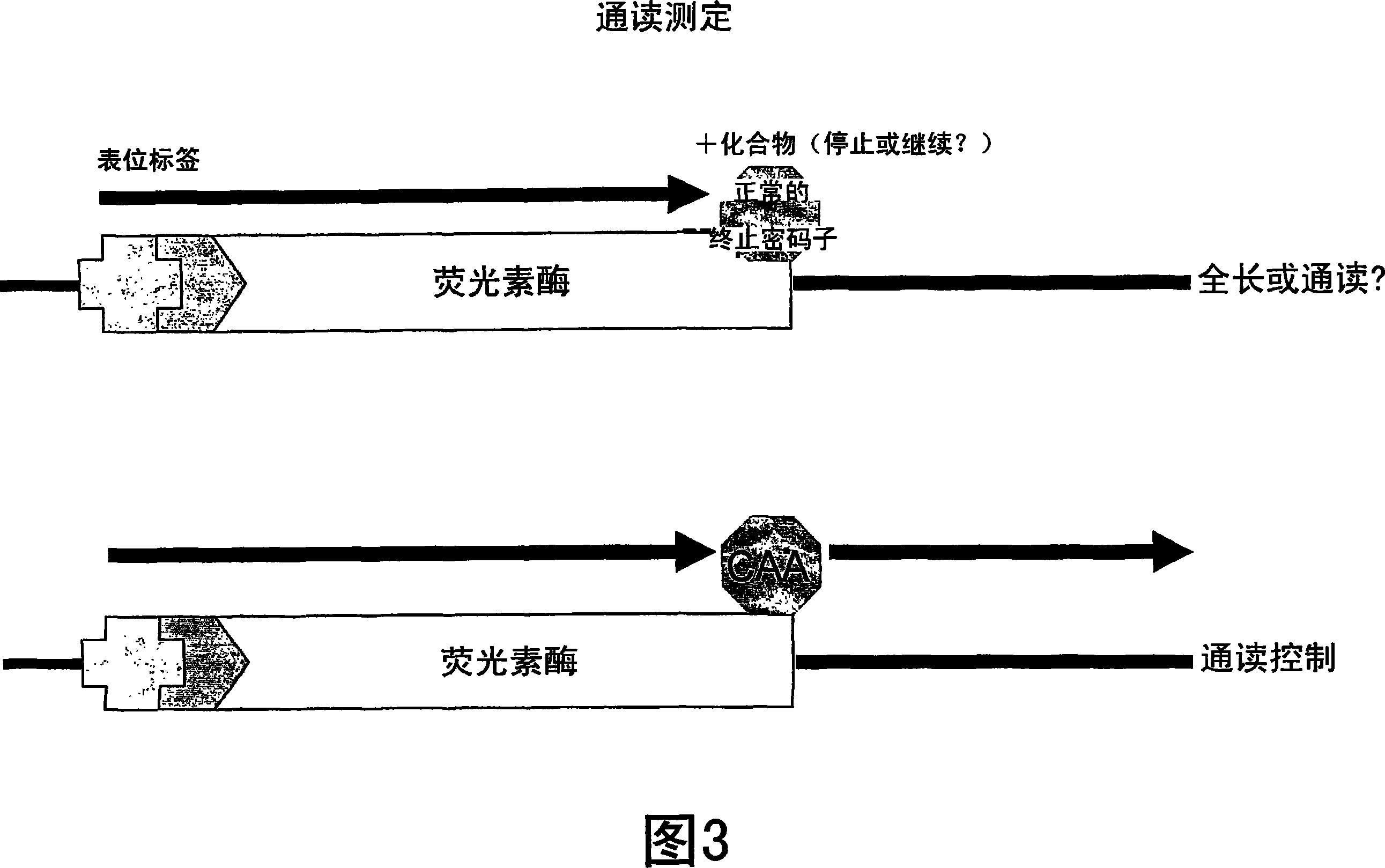
[1102] Example 2: Nonsense inhibitory activity
[1103] A functional cellular translation-based assay based on luciferase-mediated chemiluminescence (incorporated by reference in International Application PCT / US2003 / 023185, filed July 23, 2003) can evaluate normal stop codons in mRNA The translation reads through. Human embryonic kidney cells (293 cells) were cultured in medium containing fetal bovine serum (FBS). These cells were stably transfected with the luciferase gene containing a premature stop codon at amino acid 190 in place of the threonine codon (ACA) normally present in the luciferase gene at this position. One of three possible nonsense codons (TAA, TAG, or TGA) was introduced by site-directed mutagenesis, along with four possible nucleotides of an important downstream +1 site immediately following the nonsense codon ( adenine, thymine, cytosine or guanine). As such, amino acid 190 in the luciferase gene contains a premature stop codon of TAA, TAG or TGA. For ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com