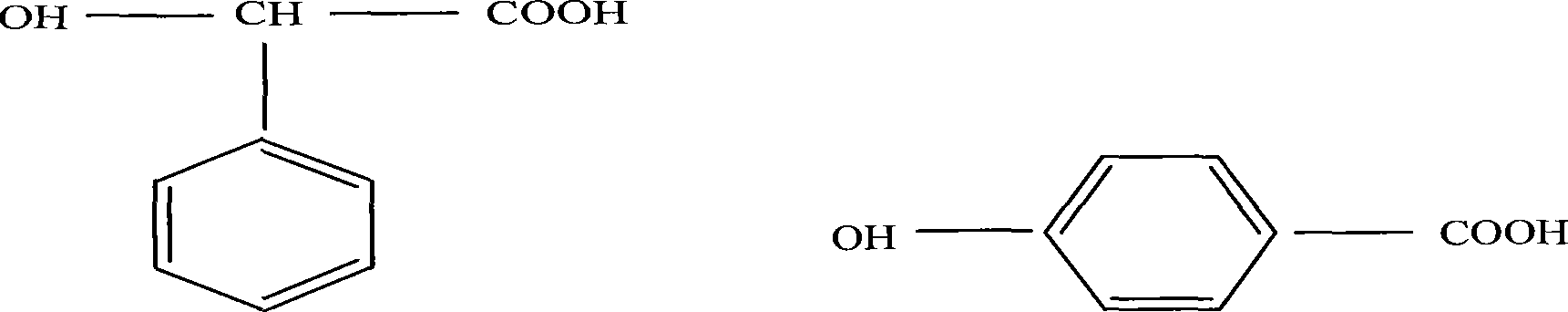Method for preparing polylactic acid-polycarbonate copolymers
A technology of polycarbonate and copolymer, which is applied in the field of preparation of polylactic acid-polycarbonate copolymer, can solve the problems of lack of flexibility and elasticity, poor toughness, high crystallinity, etc., achieve easy industrial production, improve heat resistance, The effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] (1) 368g of L-lactic acid, 20g of phenylglycolic acid and 12g of succinic acid are added to the reactor, vacuumize, add 0.5% catalyst stannous octoate to carry out polycondensation reaction, the pressure in the reactor is 2000Pa, and the temperature is 100 ℃, reacted for 5 hours, then lowered the pressure in the reactor to below 100Pa, raised the reaction temperature to 180℃, and continued to react for 20 hours to obtain M w =0.8×10 3 ~2.0×10 4 Carboxy-terminated lactic acid prepolymer;
[0022] (2) The carboxyl-terminated lactic acid prepolymer obtained in step (1) and polyhexamethylene carbonate diol (molecular weight 2000) are added in the reactor by the molar ratio of 1: 2, vacuumize, logical N 2 Gas, adding 0.1% catalyst tin protochloride to carry out polymerization reaction; Reaction temperature is 180 ℃, reacts for 10 hours, promptly obtains desired product, and this product weight-average molecular weight M w 0.5×10 5 ~1×10 5 .
Embodiment 2
[0024] (1) 736g of L-lactic acid, 40g of o-hydroxybenzoic acid and 24g of adipic acid are added to the reactor, vacuumize, add 0.8% catalyst stannous bromide to carry out polycondensation reaction, the pressure in the reactor is 1800Pa, the temperature 120°C, reacted for 3 hours, then lowered the pressure in the reactor to below 100Pa, raised the reaction temperature to 170°C, and continued to react for 15 hours to obtain M w =0.8×10 3 ~2.0×10 4 Carboxy-terminated lactic acid prepolymer;
[0025] (2) The carboxyl-terminated lactic acid prepolymer obtained in step (1) and 1,6-hexanediol carbonate (molecular weight 2000) are added in the reactor at a molar ratio of 1:1, vacuumized, and ventilated N 2 Gas, add 0.5% catalyst tin protochloride to carry out polymerization reaction; Reaction temperature is 170 ℃, reacts for 8 hours, promptly obtains desired product, and this product weight-average molecular weight M w 0.5×10 5 ~1×10 5 .
Embodiment 3
[0027] (1) 328g of L-lactic acid, 60g of o-hydroxybenzoic acid and 12g of maleic anhydride are joined in the reactor, vacuumize, add 0.1% catalyst stannous octoate to carry out polycondensation reaction, the pressure in the reactor is 1500Pa, and the temperature is 90°C, reacted for 2 hours, then lowered the pressure in the reactor to below 100Pa, raised the reaction temperature to 160°C, and continued to react for 18 hours to obtain M w =0.8×10 3 ~2.0×10 4 Carboxy-terminated lactic acid prepolymer;
[0028] (2) The carboxyl-terminated lactic acid prepolymer obtained in step (1) and polypropylene carbonate (molecular weight 2000) are added in the reactor by 1: 1 molar ratio, vacuumize, logical N 2 Gas, adding 1.5% catalyst tin protochloride to carry out polymerization reaction; Reaction temperature is 160 ℃, reacts for 6 hours, promptly obtains desired product, and this product weight-average molecular weight M w 0.5×10 5 ~1×10 5 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com