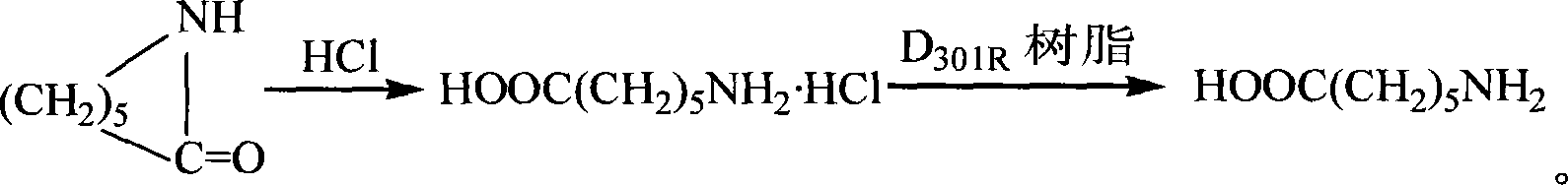Method for preparing hemostatic 6-amino caproic acid
A technology of aminocaproic acid and aminocaproic acid hydrochloride, which is applied in the preparation of organic compounds, chemical instruments and methods, and cyanide reaction preparation, can solve the problems of increased synthesis cost, cumbersome operation, and high risk, and achieve Suitable for large-scale production, mild reaction conditions, and reduced synthesis costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0010] The preparation steps of the present invention are as follows: dissolving caprolactam in 10% hydrochloric acid aqueous solution, heating to reflux, and concentrating to dryness under reduced pressure, so that hydrogen chloride does not remain in the solution to obtain 6-aminocaproic acid hydrochloride. Then the spherical macroporous weakly basic styrene-based anion exchange resin was soaked in distilled water overnight, loaded into a separation column, eluted with 0.8% aqueous ammonia solution, washed with water until neutral, and dissolved in 6-aminocaproic acid hydrochloride. Put it in water, put it into a column, elute with water, collect the eluate, concentrate it to dryness under reduced pressure, add absolute ethanol, precipitate crystals, filter, and dry to obtain 6-aminocaproic acid.
[0011] In the present invention, the weakly basic anion exchange resin is a spherical macroporous weakly basic styrene-based anion exchange resin, and its model is D370 or D301.
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com