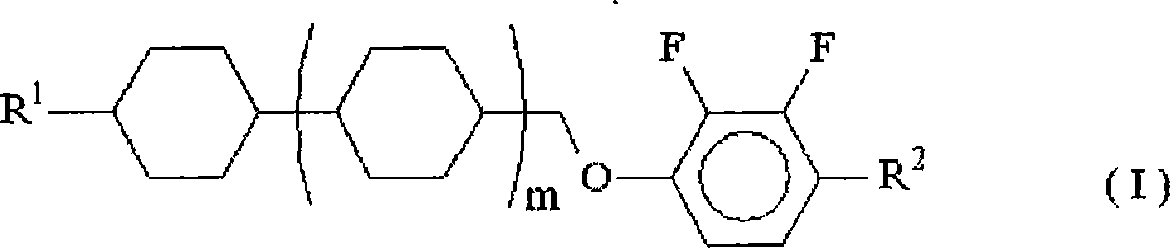
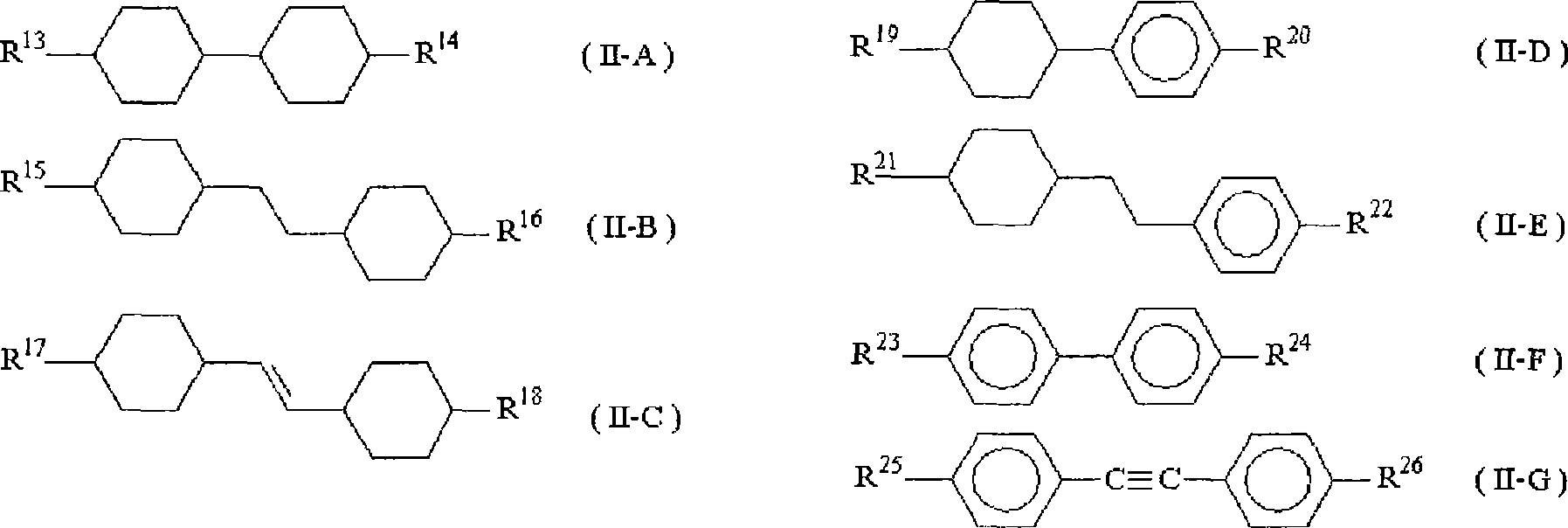
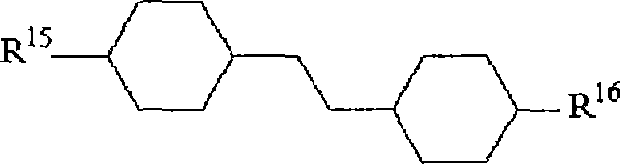
Difluorobenzene derivative and nematic liquid crystal composition by using the same
A technology of liquid crystal composition and compound, which is applied in the direction of liquid crystal materials, chemical instruments and methods, organic chemistry, etc., can solve the problems of unusable and slow liquid crystal composition for VA, and achieve high voltage retention, excellent reliability, and viscosity low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
manufacture example
[0111] (Method 1)
[0112] Make formula (9)
[0113] [chemical 15]
[0114]
[0115] The diketone compound represented is reacted with an ylide prepared from methoxymethyltriphenylsulfonium chloride to obtain formula (10)
[0116] [chemical 16]
[0117]
[0118] indicated compound. This reaction is often called the wittig reaction. The compound of the obtained formula (10) is hydrolyzed by an acid catalyst, and the compound of the formula (11) is obtained by cis-trans isomerization under alkaline conditions
[0119] [chemical 17]
[0120]
[0121] indicated compound. The resulting compound of formula (11) is reacted with an ylide prepared from methyltriphenylsulfonium bromide to obtain formula (12)
[0122] [chemical 18]
[0123]
[0124] indicated compound. The compound of gained formula (12) is reduced using reducing agents such as sodium borohydride to obtain formula (13)
[0125] [chemical 19]
[0126]
[0127] indicated compound. Gaining formula...
Embodiment 1
[0195] (Example 1) 1-((E)-2-butenyloxy)-2,3-difluoro-4-(trans-4-(trans-4-vinylcyclohexyl)cyclohexyl)methoxy Synthesis of Benzene (1a)
[0196] (1-1) Synthesis of 4-(2-butenyloxy)-2,3-difluorophenol
[0197] [chem 35]
[0198]
[0199] Synthesis of (1-1-1)1-(2-butenyloxy)-2,3-difluorobenzene
[0200] In 77.2g of 2,3-difluorophenol in 2-butanol (600ml) solution, add 122g of anhydrous potassium carbonate, then add 90.0ml of 1-bromo-2-butene (E / Z ratio=92 / 8). After heating to reflux for 4 hours, it was cooled to room temperature, and water was added dropwise to stop the reaction. It was extracted with hexane (3 times), and the collected organic layer was washed successively with 3M hydrochloric acid, water, saturated aqueous sodium bicarbonate solution, and saturated brine, and dried over anhydrous magnesium sulfate. The solvent was distilled off, and vacuum distillation (155-160° C., 50 kPa) obtained 1-(2-butenyloxy)-2,3-difluorobenzene (E / Z ratio=82) in the form of 106 ...
Embodiment 2
[0225] (Example 2) Synthesis of 2,3-difluoro-1-(2-propenyloxy)-4-(trans-4-vinylcyclohexylmethoxy)benzene (2a)
[0226] (2-1) Synthesis of 4-(2-propenyloxy)-2,3-difluorophenol
[0227] [chem 37]
[0228]
[0229] In Example 1, the same operation as (1-1) was performed using 3-bromo-1-propene instead of 1-bromo-2-butene to obtain 4-(2-propenyloxy)-2,3- Difluorophenol
[0230] (2-2) Synthesis of 2,3-difluoro-1-(2-propenyloxy)-4-(trans-4-vinylcyclohexylmethoxy)benzene
[0231] [chem 38]
[0232]
[0233] Synthesis of (2-2-1) methyl 4-methoxymethylenecyclohexanecarboxylate
[0234] Disperse 263.4 g of methoxymethyltriphenylsulfonium chloride in 750 mL of tetrahydrofuran, and add 86.2 g of potassium tert-butoxide within 5 minutes at -9 to -4°C. After stirring at -4 to -11°C for 30 minutes, 100.0 g of methyl 4-oxocyclohexanedicarboxylate was dissolved in 300 mL of THF and added dropwise at -10 to 4°C over 80 minutes. After stirring at 0-4° C. for 60 minutes, 7.0 g of ammo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com