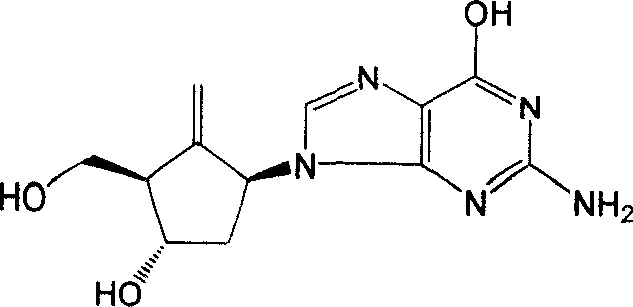
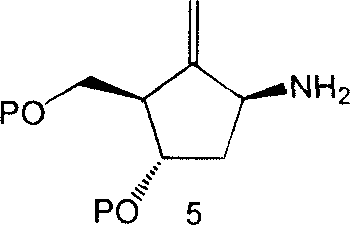
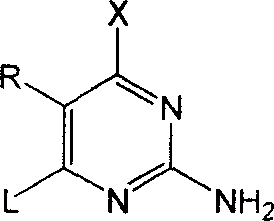
Synthesis method of antiviral nucleoside analogue
A technology of compounds and structural formulas, applied in the field of nucleoside analog synthesis, can solve the problems of difficult separation and purification of ring-opening reaction products, difficult to complete, complicated processes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Example 1: [1s-(1α, 2β, 3α, 5β)]-5-(phthalimide)-3-(benzyloxy)-2-[(benzyloxy)methyl base] cyclopentanol (intermediate 2') preparation
[0065] In a 5L three-neck flask, add 145g (0.98mol) of phthalimide, 3.77g of LiH and 765ml of anhydrous DMF, and stir for 10min. After heating to 60°C, stir for 15 minutes, at which time the cloudy solution becomes clear. Slowly drop 152g (0.49mol) [1s-(1α, 2α, 3β, 5α)]-3-(benzyloxy)-2-[(benzyloxy)methyl] dissolved in 1.87L of anhydrous DMF -6-Oxabicyclo[3.1.0]hexane (intermediate 1'), stirred at 60°C for 15min. Heated to 125°C, reacted for 2 hours, TLC (B: positive = 1:3) showed that the raw material disappeared, and terminated the reaction with 28ml of glacial acetic acid. Stir for 10 min. Add 2.5 L of saturated brine, extract with 3×1.2 L of ethyl acetate, combine the organic phases, wash once with saturated brine, dry over anhydrous sodium sulfate, and recover the solvent. The remaining oil was separated on a silica gel column ...
Embodiment 2
[0069] Example 2: [1s-(1α, 2β, 3α, 5β)]-5-[phthalimide]-3-(benzyloxy)-2-[(benzyloxy)methyl Base] the preparation of cyclopentanone (intermediate 3')
[0070] In a 3L three-necked flask, add 203g of Dess-Martin reagent, add 1.4L of anhydrous CH 2 Cl 2 Stir. 137.7g intermediate 2' was dissolved in 890ml anhydrous CH 2 Cl 2 Dissolved and added dropwise to the suspension in the previous step. After 20 min, TLC (B:positive=1:3) showed that the raw material disappeared, and the reaction was stopped. NaHSO 3 Washed three times with saturated aqueous solution, and then washed with NaHCO 3 Washed with saturated aqueous solution for 3 times, and finally washed with saturated brine for 3 times, and the organic layer was dehydrated and dried to obtain 196 g of a yellow oily compound.
Embodiment 3
[0071] Example 3: 1s-(1α, 3α, 4β)-5-phthalimido-2-methylene-4-(benzyloxy)-3-[(benzyloxy)methanol Preparation of Cyclopentane (Intermediate 4')
[0072] In a 5L three-necked flask, add Nysted Reagent (Wt=20%) 1.46L and 800ml anhydrous THF, stir, N 2 Protected and cooled to -78°C. 196g intermediate 3' with appropriate amount of CH 2 Cl 2 Dissolved and added dropwise to the reaction. Take TiCl 4 / CH 2 Cl 2 (1:9) 393ml was slowly added dropwise to the reaction, maintaining the temperature at -60°C to 78°C. After the dropwise addition was completed, the mixture was maintained at -78°C for 15 min. Slowly warm up to room temperature, and continue to stir for 1-3 h. TLC (B: positive = 1:4) showed that the raw materials disappeared, and the reaction solution was purple-black. Pour this reaction solution into 2.3L saturated NaHCO 3 In the middle, stir thoroughly, and white turbidity will appear at this time. Extracted three times with ethyl acetate, back-extracted once with s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com