Method for preparing 5-methoxy-1-(4-trifluoromethyl phenyl)pentanone
A technology of trifluoromethylphenyl and trifluoromethylbenzoic acid is applied in the field of preparation of 5-methoxy-1-(4-trifluoromethylphenyl)pentanone, which can solve the problem of environmental pollution, The actual yield is low, the volatility is strong, etc., and the production process is pollution-free, the reaction cycle is short, and the environment is friendly.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
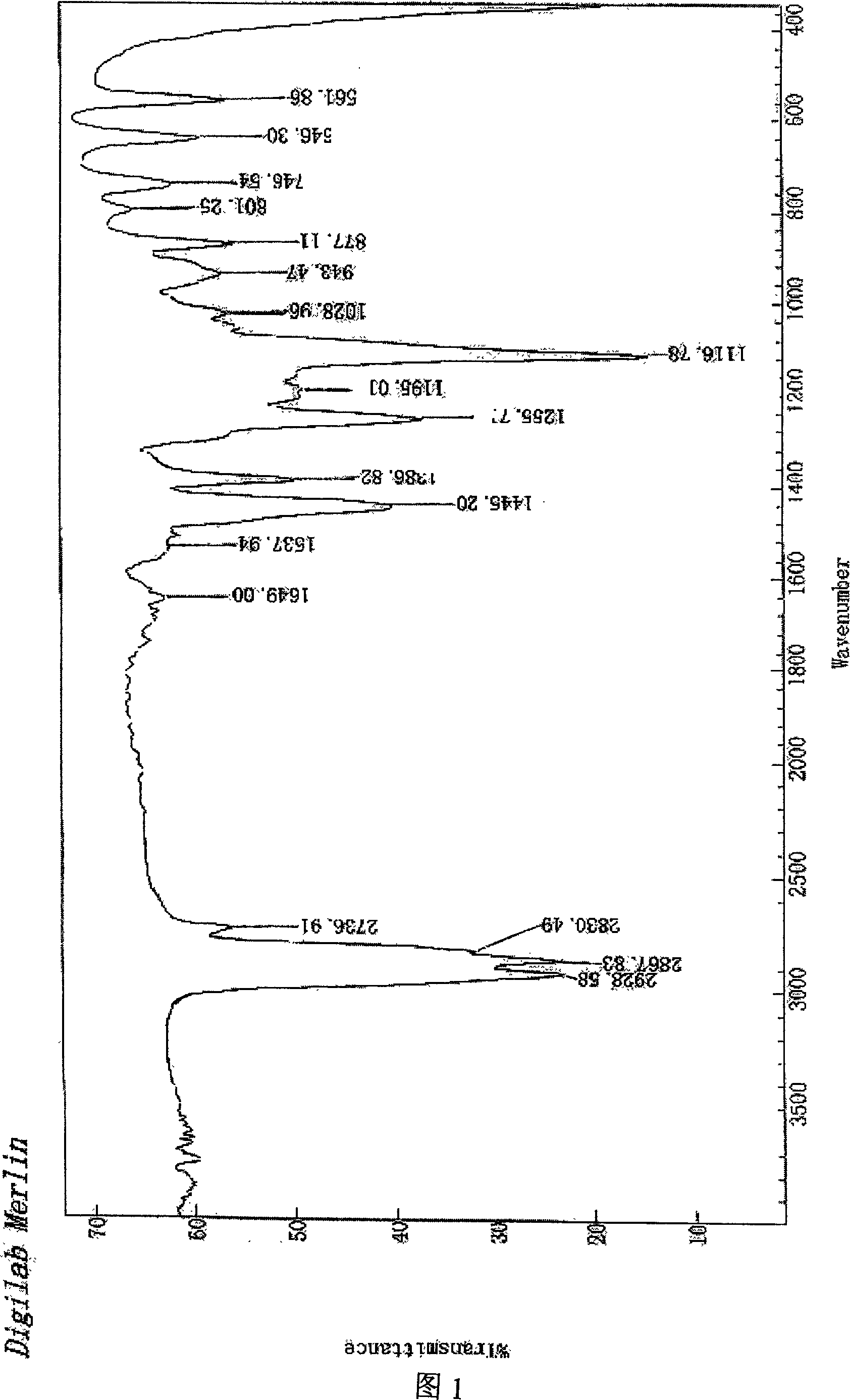
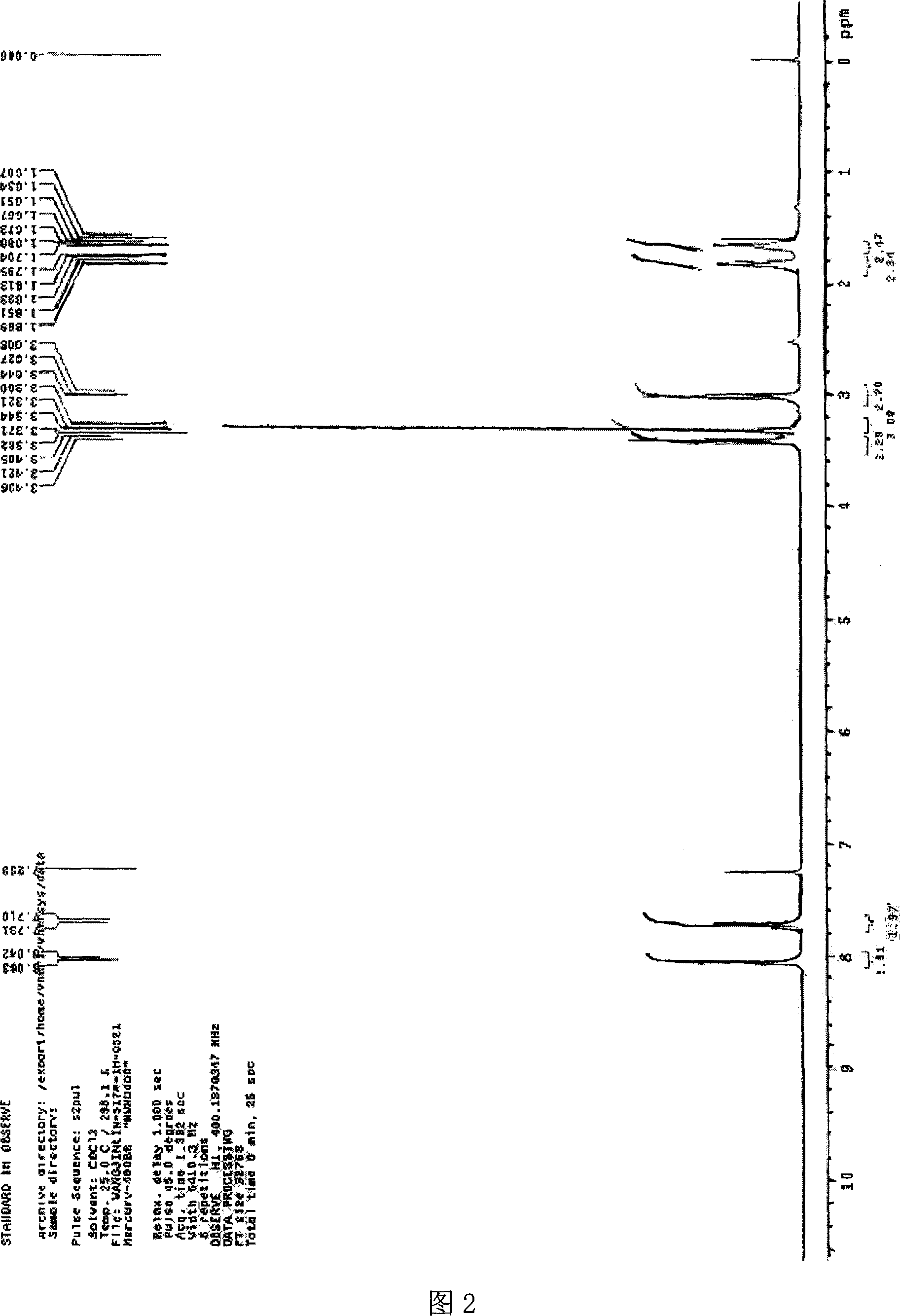
Image
Examples
Embodiment 1
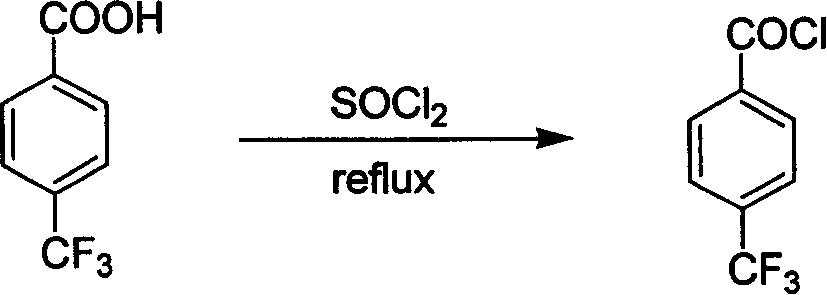
[0034] step one
[0035]
[0036] In a 50mL round-bottomed flask, add p-trifluoromethylbenzoic acid (3.81g, 20mmol), thionyl chloride (4.5g, 38mmol) and 1 drop of dimethylformamide, reflux under stirring, and wash with 40% NaOH solution Absorb the sulfur dioxide and hydrogen chloride gas produced by the reaction, and react until no gas is released. Excess thionyl chloride was recovered by distillation, and the remaining mixture was distilled under reduced pressure to collect fractions at 85-90° C. (16 mmHg) to obtain p-trifluoromethylbenzoyl chloride (3.90 g) as a colorless liquid with a yield of 93%.
[0037] step two
[0038]
[0039] Add anhydrous methanol (30mL, 0.75mol) and anhydrous tetrahydrofuran (60mL, 0.75mol) into a 250mL dry three-necked flask equipped with a condenser tube, a drying tube and a constant pressure funnel, and add thionyl chloride dropwise under cooling in an ice bath, Added within 1h.. After the addition, it was heated to reflux at 100°C for...
Embodiment 2
[0047] step one
[0048]
[0049] In a 50mL round-bottomed flask, add p-trifluoromethylbenzoic acid (3.81g, 20mmol), thionyl chloride (4.5g, 38.0mmol) and 1 drop of dimethylformamide, reflux under stirring, and The sodium solution absorbs the generated gas until the sulfur dioxide and hydrogen chloride gas disappear. Distill off the excess thionyl chloride, then distill the remaining mixture, collect 85-90 °C / 16mmHg, and obtain a colorless liquid p-trifluoromethylbenzoyl chloride (3.80g ), the yield is 91%.
[0050] step two
[0051]
[0052] Add 1,4-dibromobutane (21.5 g, 0.1 mol) and anhydrous methanol (10 ml) into a dry three-necked flask equipped with a condenser tube, a drying tube and a constant pressure funnel, stir to make it evenly mixed, and heat to After 70°C, a methanol solution of sodium methoxide (5.4 g of sodium methoxide, 30 mL of anhydrous methanol) was added dropwise. After the addition, reflux for 2 hours. After the reaction liquid is cooled, filter t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com