Polychlorinated biphenyl (PCBs) homologue semiantigen and preparation method thereof
A polychlorinated biphenyl and monochlorinated biphenyl technology is applied in the field of polychlorinated biphenyl homolog hapten and its preparation, and can solve the problems such as the preparation of polychlorinated biphenyl hapten which has not yet been seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
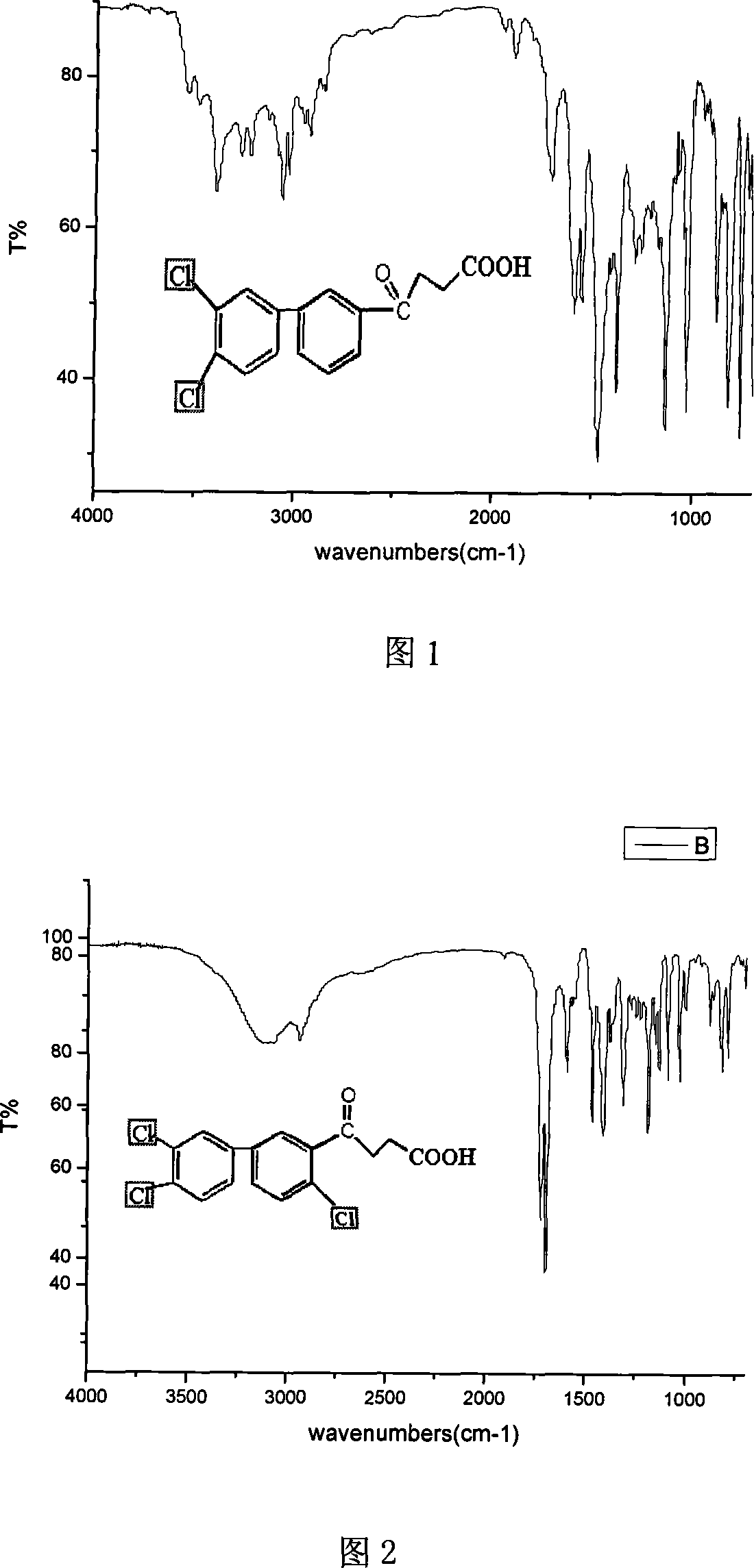
[0031] Preparation of 3,4-dichlorobiphenyl:
[0032] (1) In a 100ml beaker, warm 0.05 mol of 1,2-dichloroaniline (8.101g) and 5ml of water until the chloroaniline melts, then add 10ml of concentrated hydrochloric acid, stir vigorously, add 20ml of water, and heat until the mixture is almost completely dissolved. Then 5 ml of water was added and stirred until a homogeneous emulsion suspension was formed, at which point 3,4-dichloroaniline hydrochloride was precipitated as fine crystals. Allow to cool to room temperature, add a few pieces of small ice, cool with an ice-water bath outside, diazotize with 30% sodium nitrite at 4°C, and use pH test paper to control the acidity of the reaction to be 2-3. Endpoints were monitored with starch potassium iodide test paper.
[0033] (2) The solution after diazotization was poured into 60 ml of benzene cooled with ice water in advance, and 5 mol / L KOH solution was added dropwise at 4°C. When alkali is added, a yellow precipitation act...
Embodiment 2
[0037] Application of hapten
[0038] The polychlorinated biphenyl hapten prepared by the invention is mainly used in the immunodetection of polychlorinated biphenyls in the environment. One of its main uses is that it can be used to directly couple with protein macromolecules to prepare immunogens for immunizing animals, and then to prepare corresponding monoclonal or polyclonal antibodies. The following is an example of the use of 3,4-dichlorobiphenyl hapten:
[0039] Weigh 0.4 mmol of the hapten 4-(3,4-dichlorobiphenyl)-4-oxo-butyric acid, put it in a clean and dry small conical flask, and add 400 μL of DMF to dissolve it. 64.8 mg of DCC and 38.8 mg of NHS were weighed and dissolved in 300 μL DMF, and added dropwise to a small conical flask under magnetic stirring, and the reaction was stirred at room temperature for 8 h and overnight at 4 °C. The product after the reaction was placed in a low-temperature high-speed centrifuge at 9000 r / min, and the supernatant was separa...
Embodiment 3
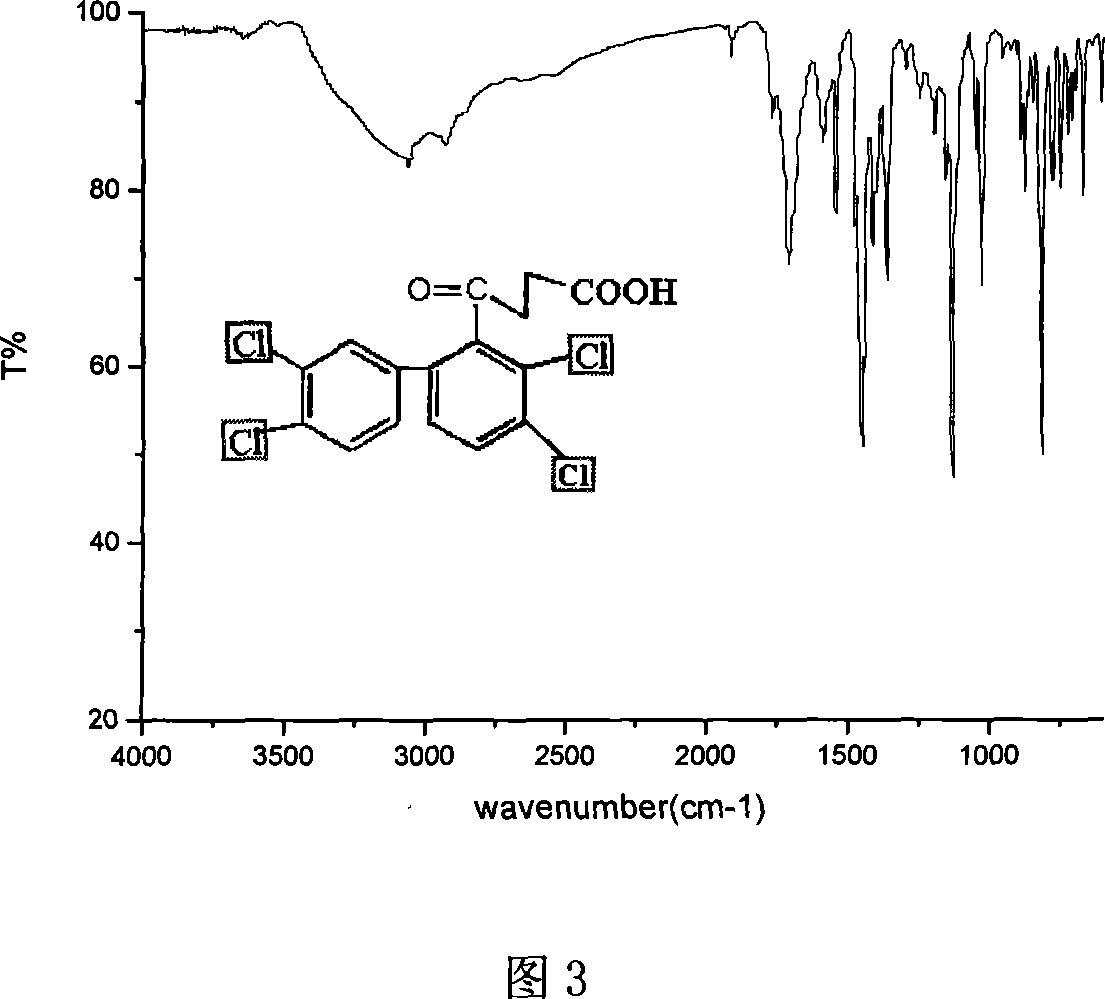
[0041] Preparation of 3,4,4'-trichlorobiphenyl and its hapten
[0042] (1) In a 100ml beaker, warm 0.05 moles of 1,2-dichloroaniline (8.101g) and 10ml of water to melt, then add 15ml of concentrated hydrochloric acid, stir, add 30ml of water, heat and stir until a uniform milky form is formed suspension. Allow to cool to room temperature, add a few pieces of small ice, cool with an ice-water bath outside, diazotize with 25% sodium nitrite at 5°C, and use pH test paper to control the acidity of the reaction to be 2-3. Endpoints were monitored with starch potassium iodide test paper.
[0043] (2) Pour the diazotized solution into 80 ml of chlorobenzene cooled with ice water in advance, and dropwise add a 5 mol / L NaOH solution at 5°C. When alkali is added, a yellow precipitation activated substance is formed, which reacts with chlorobenzene to generate 3,4,4'-trichlorobiphenyl. Reaction to strong alkaline so far. Then, the stirring was continued for 2 hours at room temperatur...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com