Chitosan alpha-aminoalkyl phosphonate ester derivative and preparation method thereof
A technology of aminoalkylphosphonic acid and chitosan, which is applied in the field of marine chemical engineering, can solve problems such as poor effect, achieve good water solubility, overcome poor solubility, and have wide application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
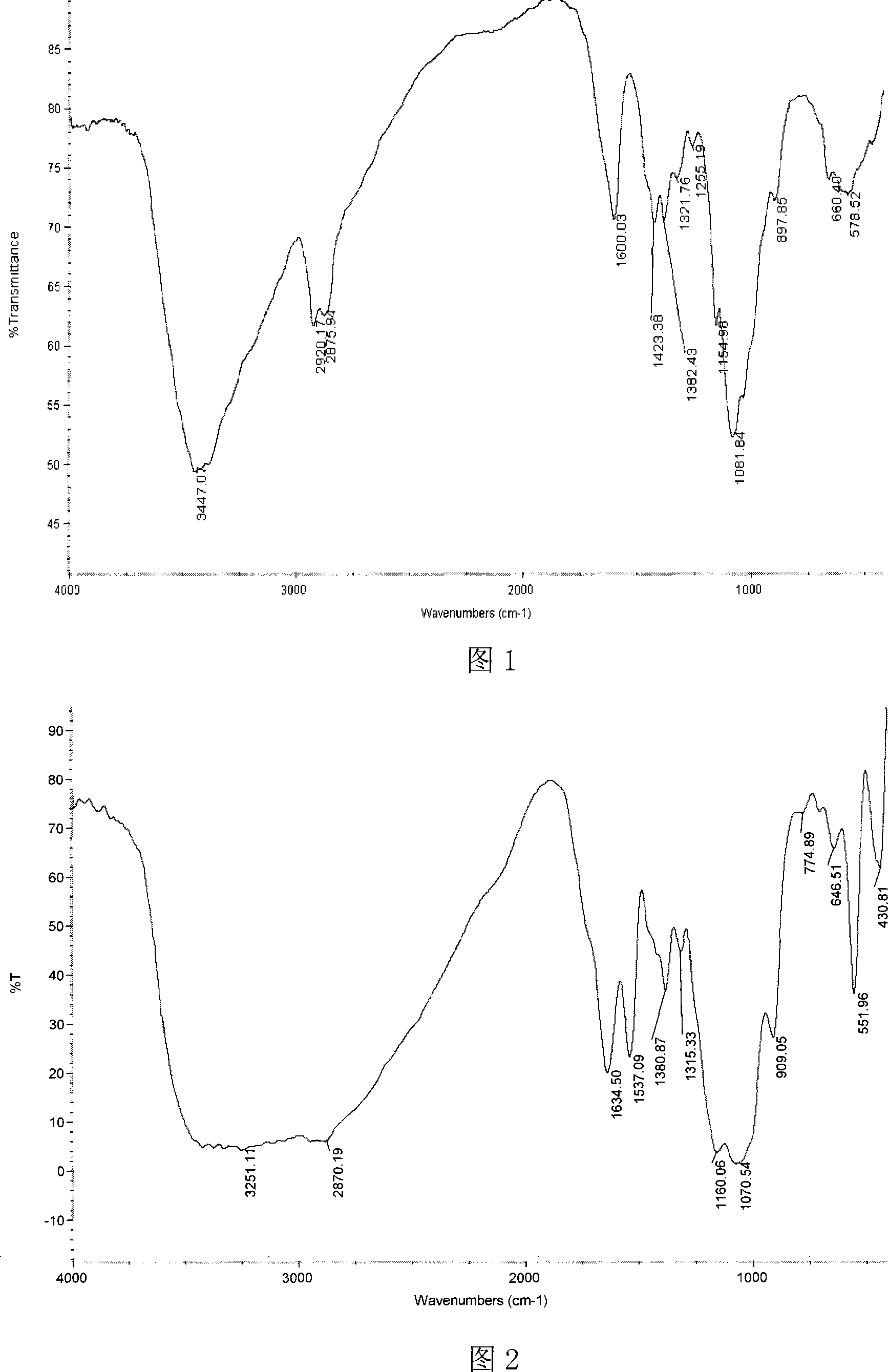
[0042] Dissolve 1.35g of chitosan propionaldehyde Schiff base with a molecular weight of 80,000 in 40mL of benzene, add 10mL of dimethyl phosphite under stirring, and react at 80°C for 6 hours. Concentrate under reduced pressure to 30 mL and cool to room temperature. Pour the reaction mixture into 300 mL of absolute ethanol to obtain a precipitate. After standing for 8 hours, filter and wash the precipitate with absolute ethanol, add 100 mL of distilled water to dissolve, and put it into a dialysis bag with a molecular weight cut-off of 3600. , dialyzed in distilled water for 2-3 days, concentrated to 31ml ± 1ml by rotary evaporation, and freeze-dried to obtain chitosan α-aminopropyl phosphonic acid dimethyl derivatives. Structural formula see formula 1, wherein R=-CH 3 , R 1 for -CH 2 CH 3 , n=49.
[0043] Infrared spectrum analysis showed that chitosan α-aminopropylphosphonic acid dimethyl ester derivatives (see Figure 2) compared with chitosan (see Figure 1): at 3447.01...
Embodiment 2
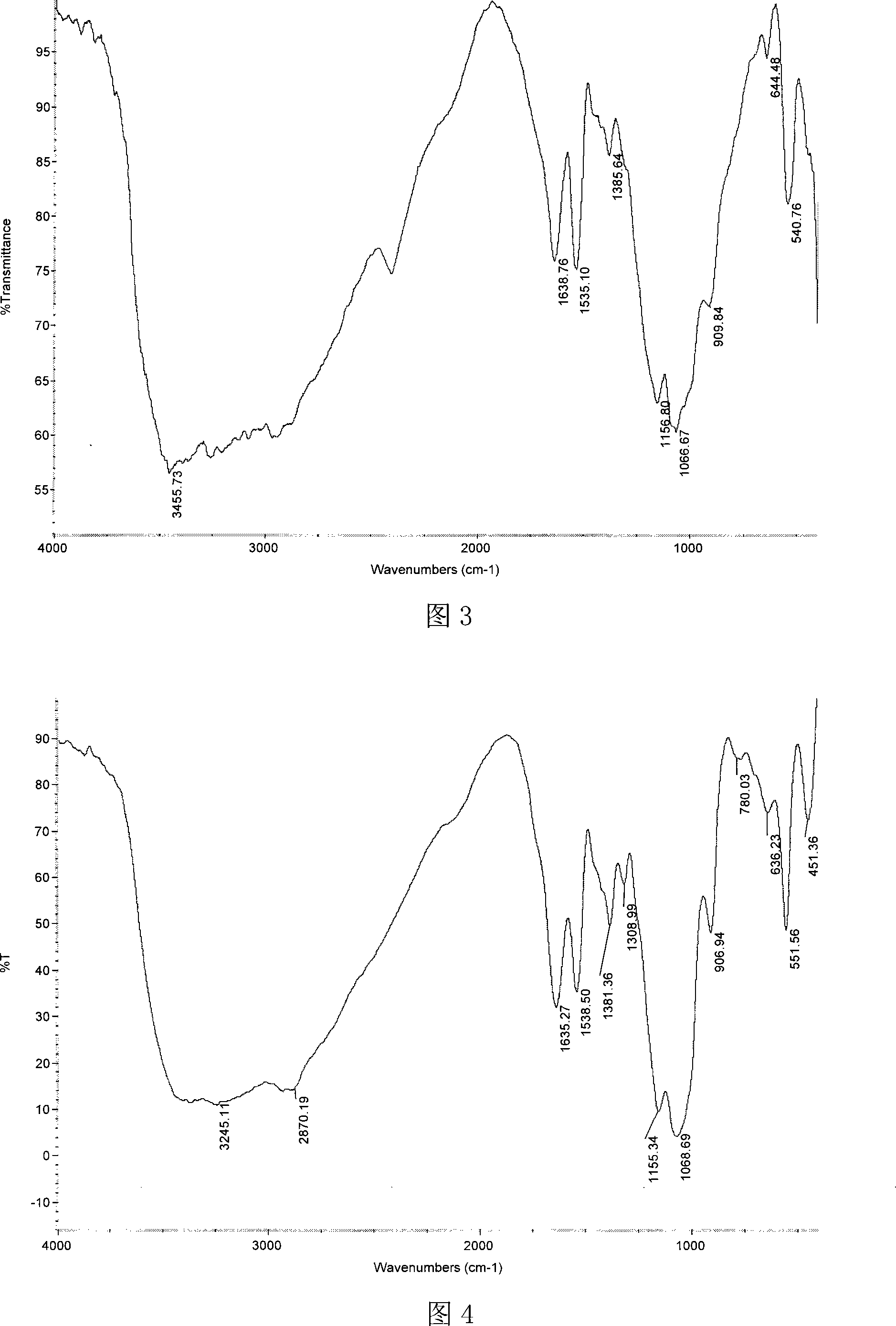
[0045] Dissolve 1.51g of chitosan propionaldehyde Schiff base with a molecular weight of 500,000 in 60mL of benzene, add 10mL of dimethyl phosphite under stirring, and react at 90°C for 7 hours. Concentrate under reduced pressure to 40 mL and cool to room temperature. Pour the reaction mixture into 300 mL of absolute ethanol to obtain a precipitate. After standing for 8 hours, filter and wash the precipitate with absolute ethanol, add 100 mL of distilled water to dissolve, and put it into a dialysis bag with a molecular weight cut-off of 3300. , dialyzed in distilled water for 2-3 days, concentrated to 35ml ± 1ml by rotary evaporation, and freeze-dried to obtain chitosan α-aminopropylphosphonic acid dimethyl ester derivatives. Structural formula see formula 1, wherein R=-CH 3 , R 1 for -CH 2 CH 3 , n=3106.
[0046] Infrared spectrum analysis shows that chitosan α-aminopropyl phosphonic acid dimethyl ester derivative (see Figure 3) is compared with chitosan (see Figure 1): ...
Embodiment 3
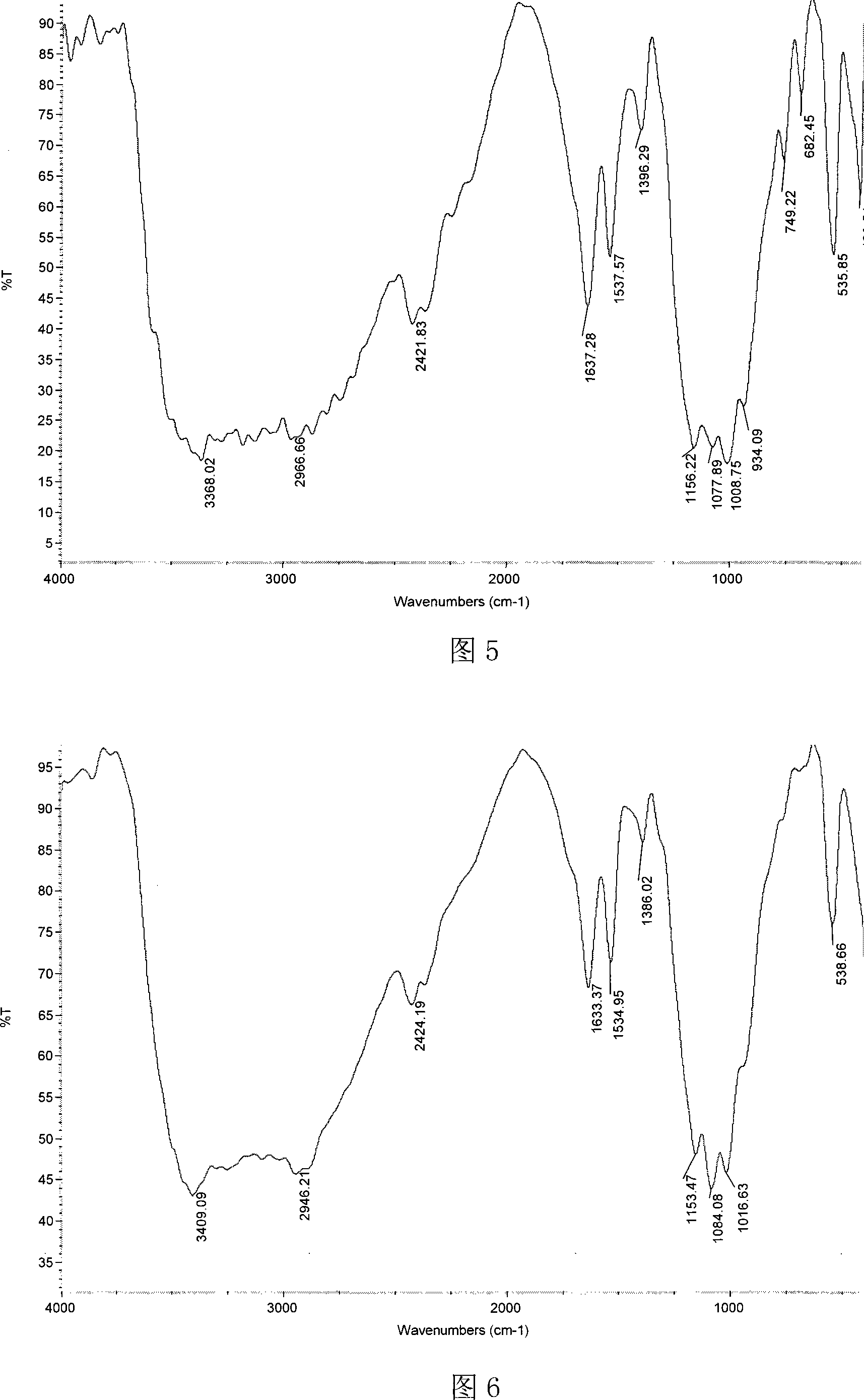
[0048] Dissolve 1.79g of chitosan propionaldehyde Schiff base with a molecular weight of 20,000 in 40mL of benzene, add 10mL of diethyl phosphite under stirring, and react at 90°C for 6 hours. Concentrate under reduced pressure to 35 mL and cool to room temperature. Pour the reaction mixture into 300 mL of absolute ethanol to obtain a precipitate. After standing for 8 hours, filter and wash the precipitate with absolute ethanol, add 100 mL of distilled water to dissolve, and put it into a dialysis bag with a molecular weight cut-off of 3400. , dialyzed in distilled water for 2-3 days, concentrated to 39ml ± 1ml by rotary evaporation, and freeze-dried to obtain chitosan α-aminopropylphosphonic acid diethyl ester derivatives. Structural formula see formula 1, wherein R=-CH 2 CH 3 , R 1 for -CH 2 CH 3 , n=124.
[0049] Infrared spectrum analysis shows that chitosan α-aminopropyl phosphonic acid diethyl ester derivative (see Figure 4) is compared with chitosan (see Figure 1):...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com