Method for preparing phenylacetic acid by non-catalyzed hydrolysis of benzene acetonitrile in near-critical water medium
A near-critical water, catalytic hydrolysis technology, applied in the field of carboxylic acids, can solve the problems of complex process flow, imperfect catalyst preparation, selection and recovery, etc., achieve simple reaction process, high product purity and yield, and improve yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
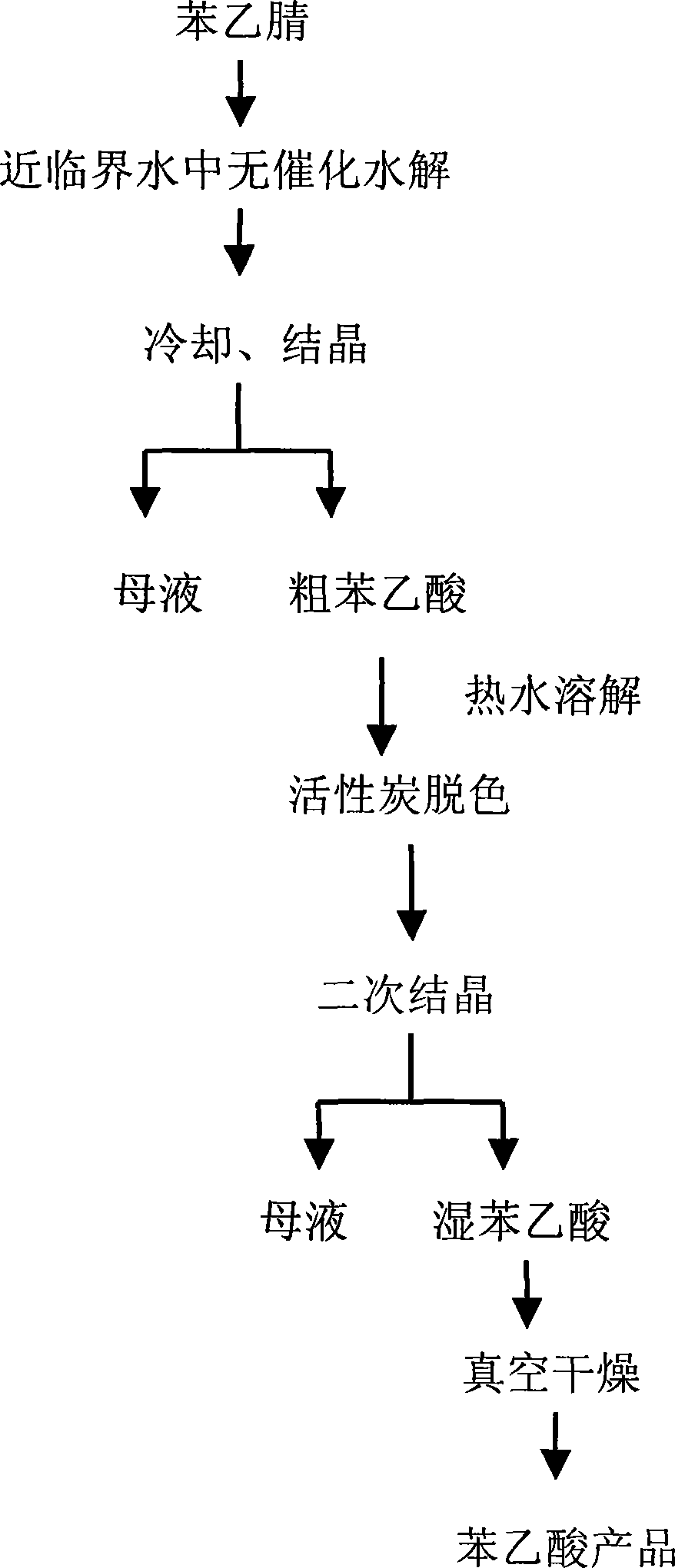
[0023] Add 340g of deionized water and 170g of phenylacetonitrile (the mass ratio of deionized water to phenylacetonitrile is 2:1) in a 500mL intermittent autoclave, start stirring, heat up to boiling under normal pressure, open the exhaust valve for 5min, and use water Remove the air in the kettle by steam; close the exhaust valve, and continue to heat up to 240 ° C for 8 hours; the reaction product is cooled and crystallized to obtain crude phenylacetic acid. The crude product of phenylacetic acid was decolorized by activated carbon, recrystallized and dried in vacuum to obtain 139.1 g of phenylacetic acid product, the product was analyzed by HPLC with a purity of 98.4% (wt%) and a yield of 81.8%.
Embodiment 2
[0025] Add 360g of deionized water and 120g of phenylacetonitrile (the mass ratio of deionized water to phenylacetonitrile is 3:1) into a 500mL intermittent autoclave, start stirring, heat up to boiling under normal pressure, open the exhaust valve for 2min, and use water Remove the air in the kettle by steam; close the exhaust valve, and continue to heat up to 250°C for 7 hours; the reaction product is cooled and crystallized to obtain a crude product of phenylacetic acid, which is decolorized with activated carbon, recrystallized, and vacuum-dried to obtain 96.96g of phenylacetic acid product, which is tested by HPLC The analytical purity was 98.7% (wt%) and the yield was 80.8%.
Embodiment 3
[0027] Add 360g of deionized water and 60g of phenylacetonitrile (the mass ratio of deionized water to phenylacetonitrile is 6:1) into a 500mL intermittent autoclave, start stirring, heat up to boiling under normal pressure, open the exhaust valve for 4min, and use water Remove the air in the kettle by steam; close the exhaust valve, and continue to heat up to 250°C for 7 hours; the reaction product is cooled and crystallized to obtain a crude product of phenylacetic acid. The crude product is decolorized with activated carbon, recrystallized, and vacuum-dried to obtain 45.96g of phenylacetic acid product. The product is tested by HPLC The analytical purity was 99.1% (wt%) and the yield was 76.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com