Artificial cerebrospinal fluid
An artificial, aqueous solution technology, applied in the field of artificial spinal fluid, can solve the problems of no clarification of the relationship between cleaning fluid or perfusion fluid cerebral edema, no report, and reducing the composition of cleaning fluid, etc., to achieve the effect of inhibiting ion exchange damage and reducing the risk rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
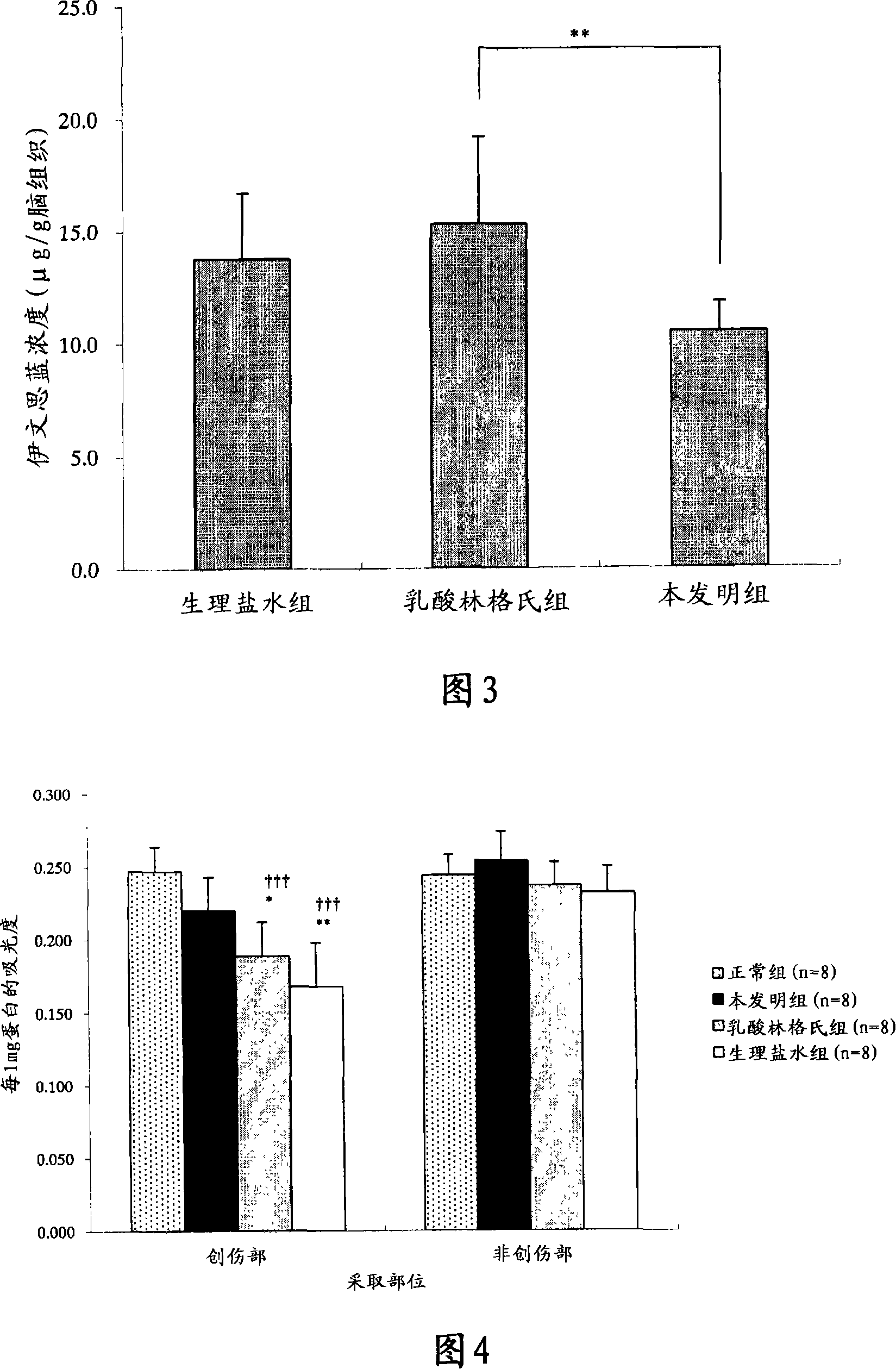
[0118] The components shown in the following Table 1 as the upper chamber liquid and the lower chamber liquid were weighed, respectively mixed and dissolved in distilled water for injection to prepare 150 mL of the upper chamber liquid and 350 mL of the lower chamber liquid.
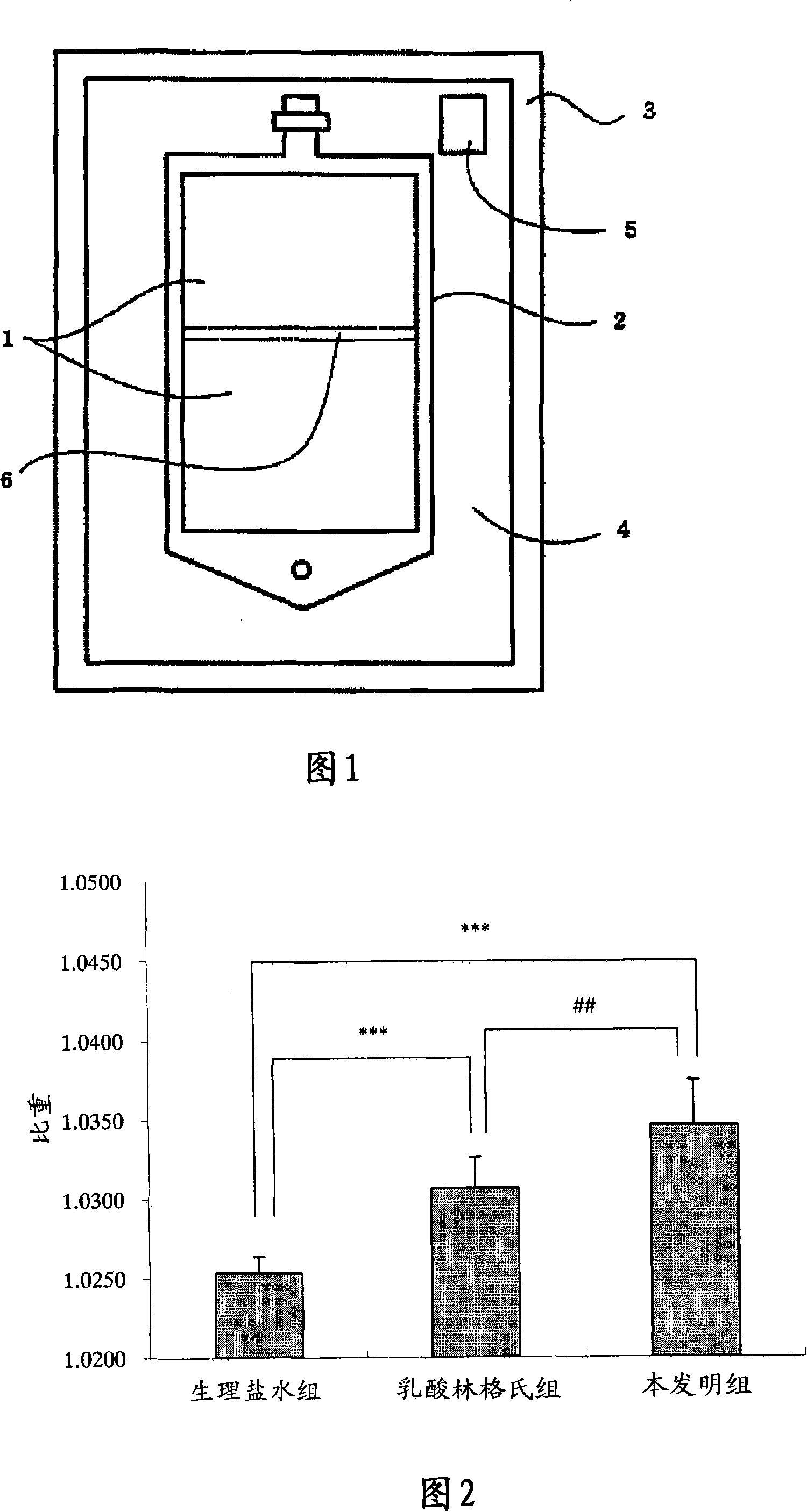
[0119] The obtained lower chamber fluid is filled into the lower chamber (chamber with a liquid outlet connected to the exhaust port) of the container 2 (made of polyethylene) of the container package for storing artificial spinal fluid shown in FIG. 1, depicted as the upper part), seal the mouth. In addition, the obtained upper chamber liquid was filled in the upper chamber before sealing (the chamber separated from the above-mentioned lower chamber by the partition wall, the chamber adjacent to the hanger part, drawn as the lower part in the figure) and sealed.
[0120] Table 1
[0121] Element
amount (g)
Upper chamber solution (per 150ml)
Magn...
Embodiment 2
[0127] Except for removing glucose from the upper chamber fluid described in Example 1, the same procedure as in Example 1 was performed to prepare a container package for accommodating the artificial spinal fluid of the present invention, and the same experiment was carried out. The obtained results are shown in Table 3 below.
[0128] table 3
[0129] ingredients
Embodiment 3
[0131] Except for removing potassium dihydrogen phosphate from the lower chamber fluid described in Example 1, the same procedure as in Example 1 was performed to prepare a container package for accommodating the artificial spinal fluid of the present invention, and the same experiment was carried out. The obtained results are shown in Table 4 below.
[0132] Table 4
[0133] ingredients
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com