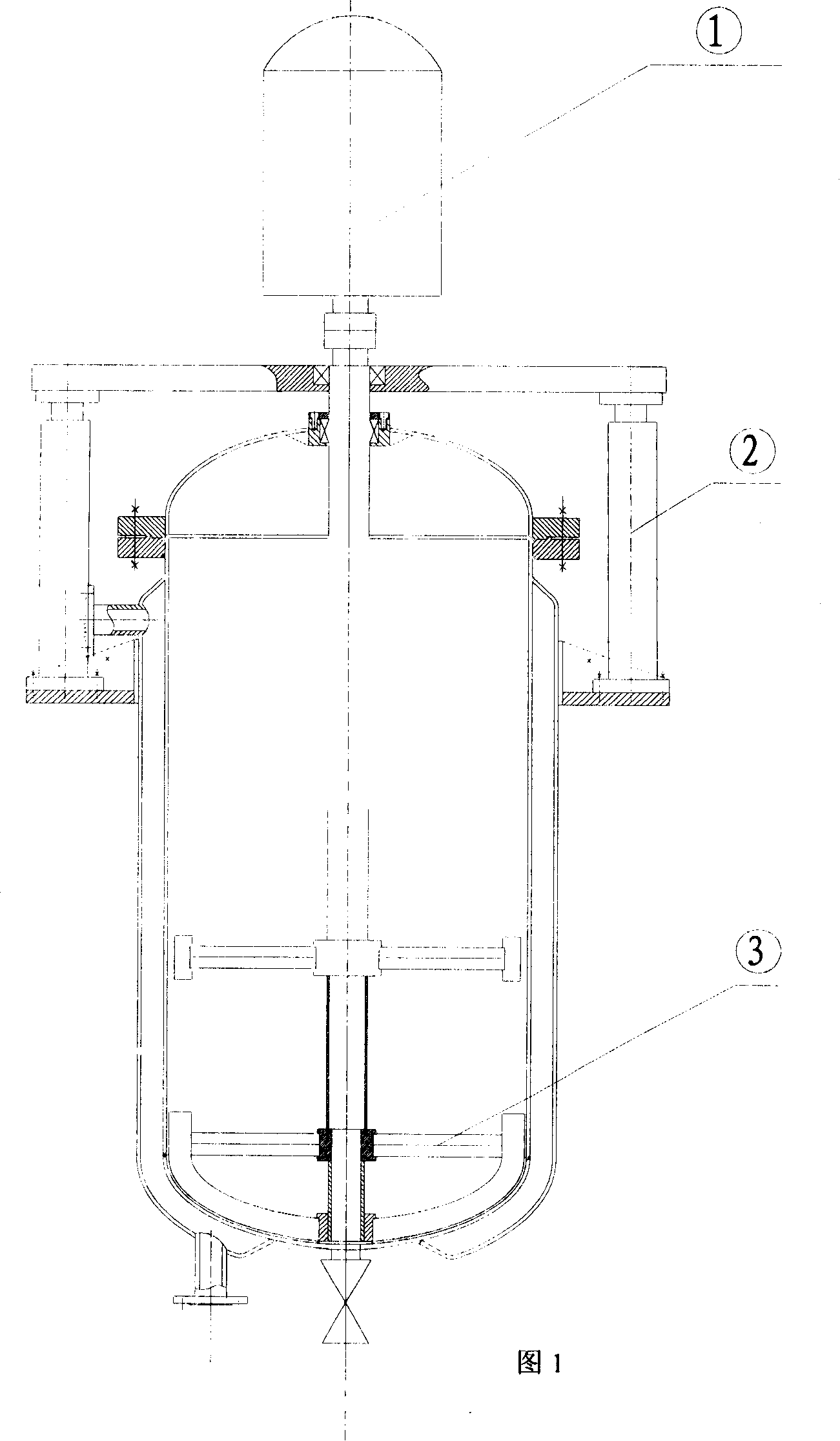
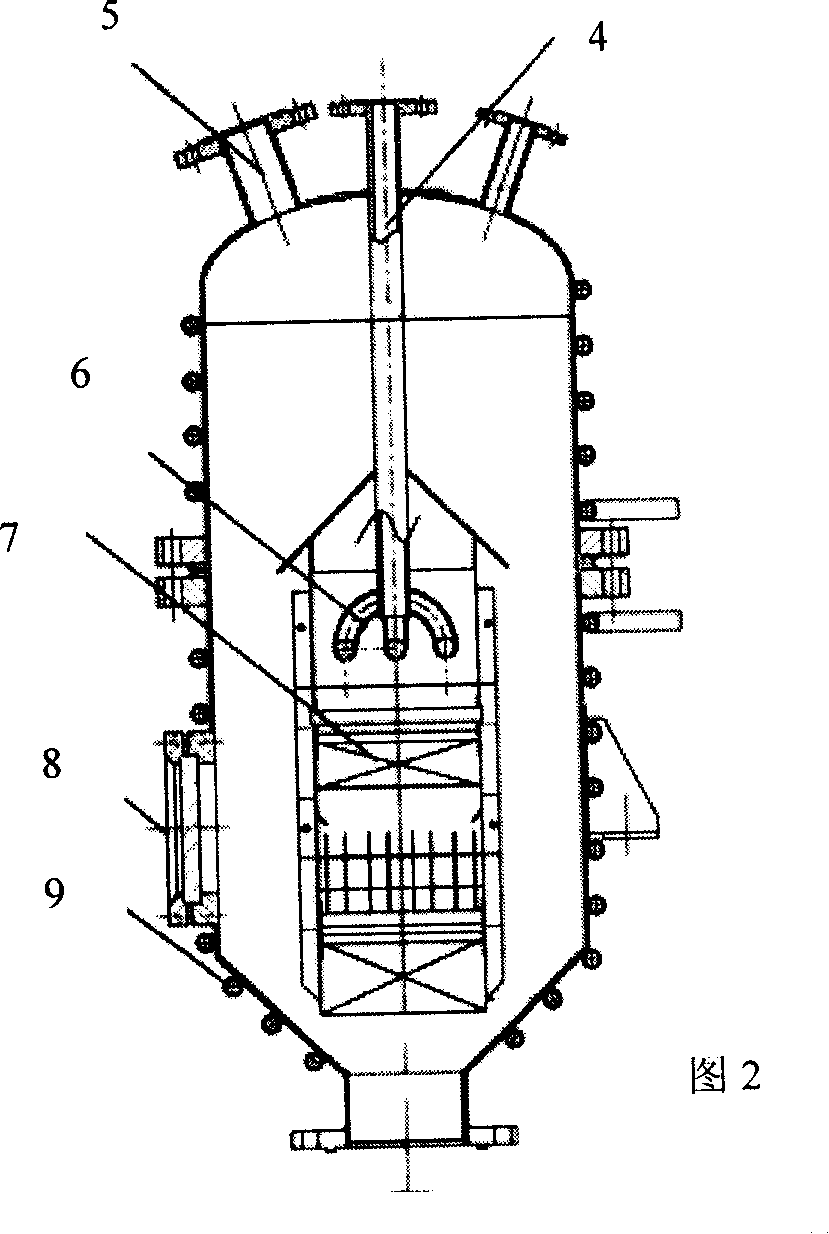
Method for preparing polyaramide resin modified by aromatic heterocycle and chloromonomer
A technology of polyaramid and manufacturing method, which is applied in the field of special synthetic fiber production, and can solve problems such as difficult molecular weight, difficult recycling, and high price of lithium chloride
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1: be 97% NMP and 17.7 parts of content be the CaCl of 35% by 100 parts (weight) content 2 After mixing evenly, heat it to 200°C through a preheater, and then input it into a rectification tower to remove water, and obtain 103.2 parts of CaCl 2 A 6% NMP solution (water content≤150ppm) is used as a solvent. Then 40% moles of 2-5'dichloro-p-phenylenediamine and 60% moles of 2-(4aminophenyl)-5(6)aminophenylbenzimidazole were dissolved in 6% (by weight) CaCl 2 in NMP solvent (the concentration of diamine in NMP is 5%). Then the dissolved diamine solution is cooled to 2-5° C., and 50% mole of terephthaloyl chloride is added under vigorous stirring to form a low-molecular-weight oligomer terminated by an amino group. Then cool the low-molecular-weight oligomer to 2-5 DEG C, and gradually add the remaining 50% by mole of terephthaloyl chloride to obtain a resin solution with a kinetic viscosity of 700 poise.
[0019] After the resin solution is filtered, it is de...
Embodiment 2
[0024] Example 2: CaCl 2 The preparation method of the NMP solution (water content≤150ppm) with a content of 6% is the same as in Example 1.
[0025] 40% mole 2-chloro-p-phenylenediamine and 60% mole 2-(4 aminophenyl)-5(6) aminophenylbenzimidazole are dissolved in 6% (weight) CaCl by the method of embodiment 1 2 in the NMP solvent. Then the dissolved diamine solution is cooled to 2-5° C., and 50% mole of terephthaloyl chloride is added under vigorous stirring to form a low-molecular-weight oligomer terminated by an amino group. Then cool the low-molecular oligomer to 2-5 DEG C, and gradually add the remaining 50% mole terephthaloyl chloride to obtain a resin solution with a kinetic viscosity of 900 poise.
[0026] The properties of the fiber obtained after the resin solution is degassed and spun in a falling film evaporator are as follows:
[0027] Single filament strength 6.0GPa
[0028] The relative elongation at break is 1.9%;
[0029] Elastic modulus 172GPa,
[0030]...
Embodiment 3
[0031] Example 3: CaCl 2 The preparation method of the NMP solution (water content≤150ppm) with a content of 6% is the same as in Example 1.
[0032] 80% mole 2-chloro-p-phenylenediamine and 20% mole 2-(4 aminophenyl)-5(6) aminophenyl benzimidazole are dissolved in 6% (weight) CaCl by the method of embodiment 1 2 in the NMP solvent. Then the dissolved diamine solution is cooled to 2-5° C., and 50% mole of terephthaloyl chloride is added under vigorous stirring to form a low-molecular-weight oligomer terminated by an amino group. Then cool the low-molecular oligomer to 2-5 DEG C, and gradually add the remaining amount of 50% mole terephthaloyl chloride to obtain a resin solution with a dynamic viscosity of 800 poise.
[0033] The properties of the fiber obtained after the resin solution is degassed and spun in a falling film evaporator are as follows:
[0034] Single filament strength 6.0GPa
[0035] The relative elongation at break is 1.6%;
[0036] Elastic modulus 180GPa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Dynamic viscosity | aaaaa | aaaaa |
| Monofilament strength | aaaaa | aaaaa |
| Elastic modulus | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com