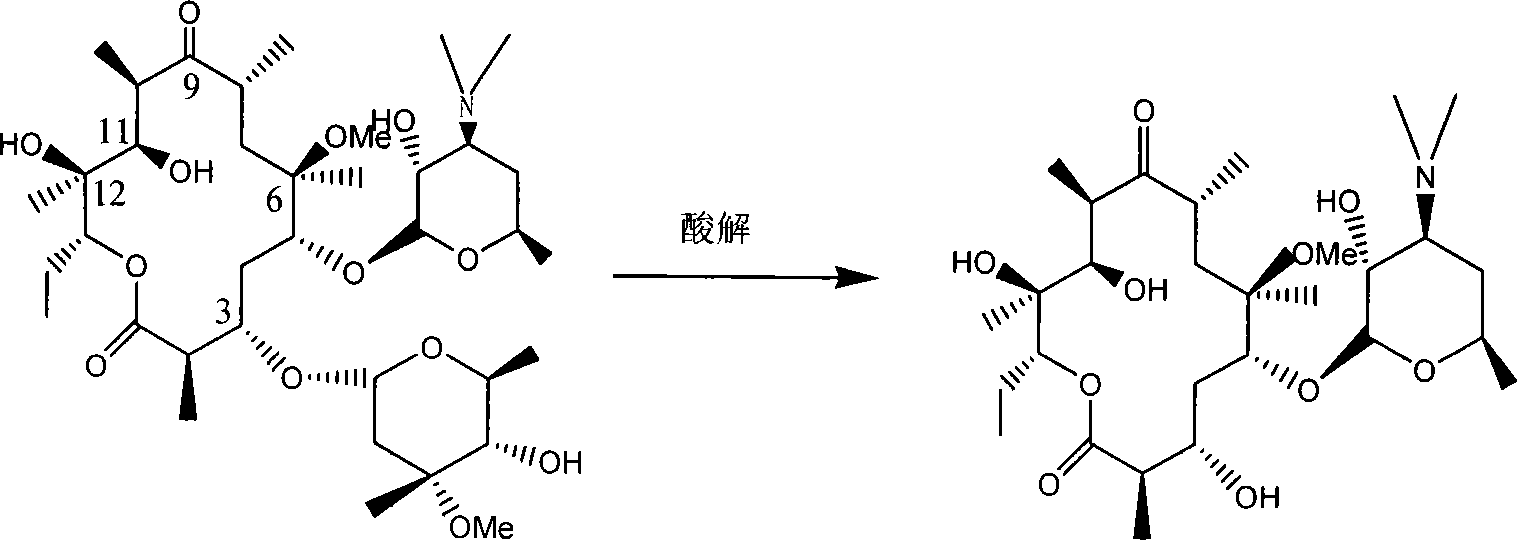
Method for purifying 3-hydroxyclarithromycin
A technology of hydroxy clarithromycin and a purification method, which is applied in the field of purification of 3-hydroxy clarithromycin, can solve the problems of inability to obtain hydrolyzed products and reduced yield, and achieve the effects of high yield, short reaction time and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 3g of clarithromycin, 80ml of water and 2.4m of 138% concentrated hydrochloric acid, stirred at 25°C for 120min; added dropwise ammonia water to adjust the pH value to 9.0, added sodium chloride until the solution was saturated, extracted with 30ml×3 ethyl acetate: washed with saturated saline; Filtration, distillation under reduced pressure; Recrystallization in acetone / petroleum ether system; Filtration, dry to obtain white needle-like crystal 2.12g (yield 89.7%, purity 95%) at last
Embodiment 2
[0029] 3g of clarithromycin, 80ml of water and 2.4ml of 38% concentrated hydrochloric acid, stirred at 25°C for 120min; added dropwise ammonia water to adjust the pH value to 9.0, white flocculent precipitates precipitated; added sodium chloride until the solution was saturated, and precipitated; suction filtered , washed the filter cake with saturated brine; dried the filter cake, dissolved in ethyl acetate, suction filtered, and dried over anhydrous magnesium sulfate for 12 hours; distilled under reduced pressure, and dried at 40°C to obtain 1.87g of white needle-shaped crystals (yield 79.0%, purity 96.5%)
Embodiment 3
[0031] Mix 2.0g of clarithromycin, 53ml of distilled water and 1.0ml of 38% hydrochloric acid, stir at 30°C for 90min; add dropwise ammonia water to adjust the pH value to 7.0, extract with 15ml ethyl acetate; add dropwise ammonia water to adjust the pH value of the extracted aqueous phase To 8.0, add sodium chloride to saturation, and extract again with 15ml of ethyl acetate × 3; wash twice with saturated sodium bicarbonate solution, twice with saturated sodium chloride solution; dry over anhydrous magnesium sulfate; filter with suction, and distill under reduced pressure; Recrystallize in acetone / petroleum ether system; filter and dry to obtain 1.39g of white needle-like crystals (yield 88%, purity 99.0%)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com