Micrococcus pyogenes adhesion functional peptide and coded sequence thereof
A staphylococcus, golden yellow technology, applied in the direction of peptides, depsipeptides, antibacterial drugs, etc., can solve problems such as danger and limitation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Materials and methods
[0049] Strains and plasmids: The clinical isolates of Staphylococcus aureus cow mastitis were isolated and identified in a conventional manner; Escherichia coli BL21(DE3); pMD18-T vector was purchased from TaKaRa; pET32a + Vectors were purchased from Novagen.
[0050] Reagents: high-fidelity DNA polymerase, restriction endonucleases (BamH I and HindIII), T 4 DNA ligase was purchased from TaKaRa; DNA gel recovery kit was purchased from U-gene; ampicillin was purchased from Huamei Company; Ni-NTA affinity chromatography column was a product of QIAGEN.
[0051] Experimental animals: Kunming mice, weighing 18-22 g, purchased from the Experimental Animal Center of Shandong University of Traditional Chinese Medicine.
Embodiment 2
[0053] PCR amplification of Fnbp gene and its sequencing analysis
[0054] The DNA template of Staphylococcus aureus was extracted by phenol-chloroform method. According to the Fnbp gene sequence published on GenBank, a pair of primers were designed and synthesized:
[0055] Upstream (including BamH I restriction site): KF1: 5′-AAA GGATCC GCATCAGAACAAAAGACA-3';
[0056] Downstream (including Sal I restriction site): KF2: 5′-AGA GTC GAC CTACTCAGAGGACTCAGTGTA-3'.
[0057] 50μL PCR reaction system: 5μL 10×PCR buffer, Mg 2+ (25mM) 3μL, dNTP (25mM) 4μL, Taq enzyme (2.5U) 0.5μL, each primer 1μL, template 2μL, sterile double distilled water 33.5μL. Cycle conditions: 94°C pre-denaturation for 5 minutes; 94°C for 1 minute, 50°C for 1 minute, 72°C for 3 minutes, 30 cycles; 72°C for 10 minutes.
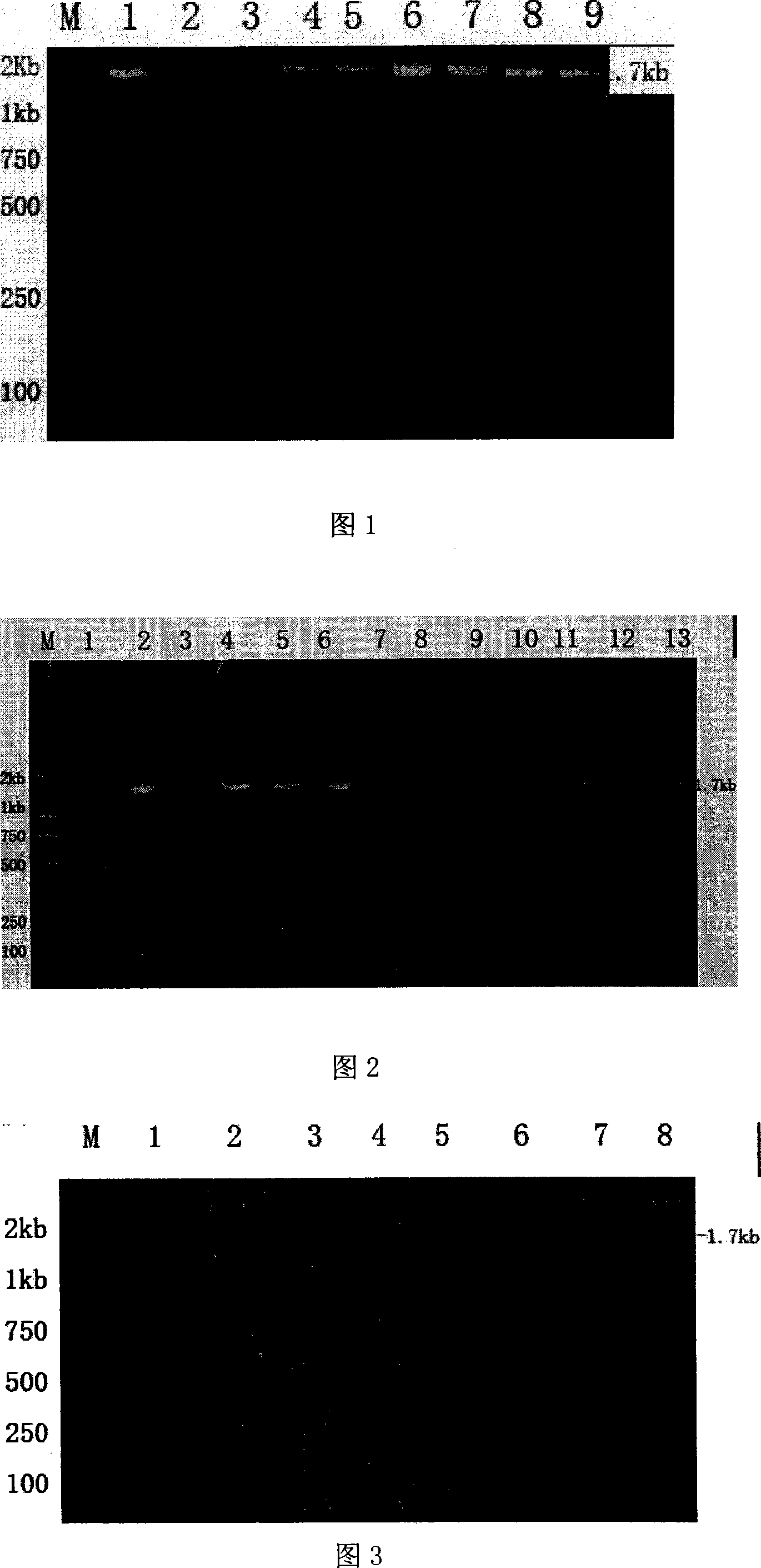
[0058] The PCR product was connected with the pMD18-T vector, transformed into E.coli DH5α, and the extracted plasmid was identified by agarose electrophoresis after double digestion with...
Embodiment 3
[0060] Construction of recombinant expression plasmids
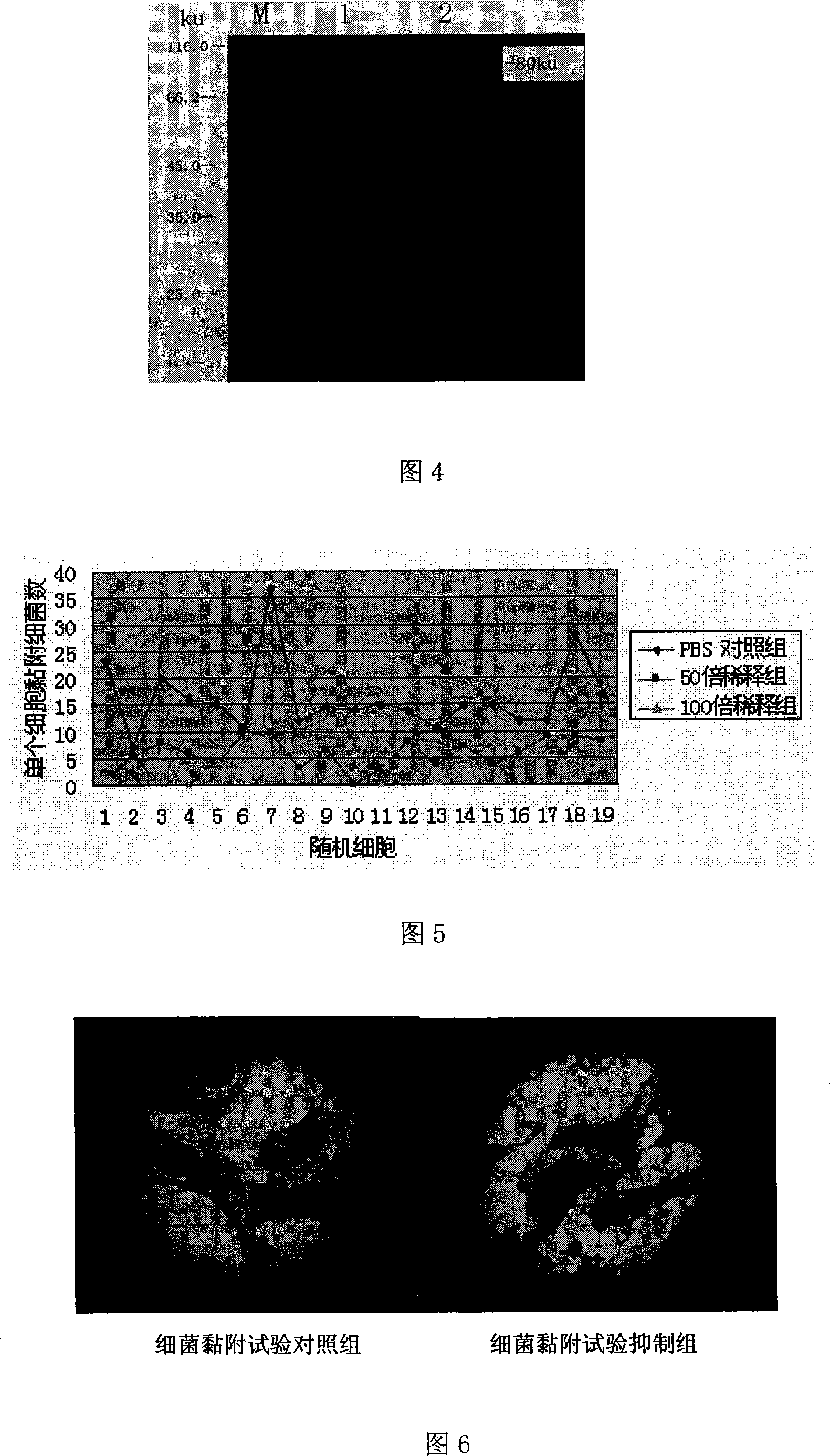
[0061] Extract the positive plasmid in Example 2, after double digestion with BamH I and Sal I, use the kit to recover the target fragment, and pET-32a that has also been double digested with BamH I and Sal I + The expression vector was connected to construct the recombinant expression plasmid pET32a + -Fnbp, and carry out BamH I, Sal I double enzyme digestion identification and PCR identification on the recombinant plasmid. After double enzyme digestion, there was a specific band at about 1.7kb, and a specific band was amplified at about 1.7kb through PCR identification, indicating that the recombinant plasmid was constructed correctly (see Figure 2 and 3 for the identification results).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com