Establishment and application of novel E-LAMP
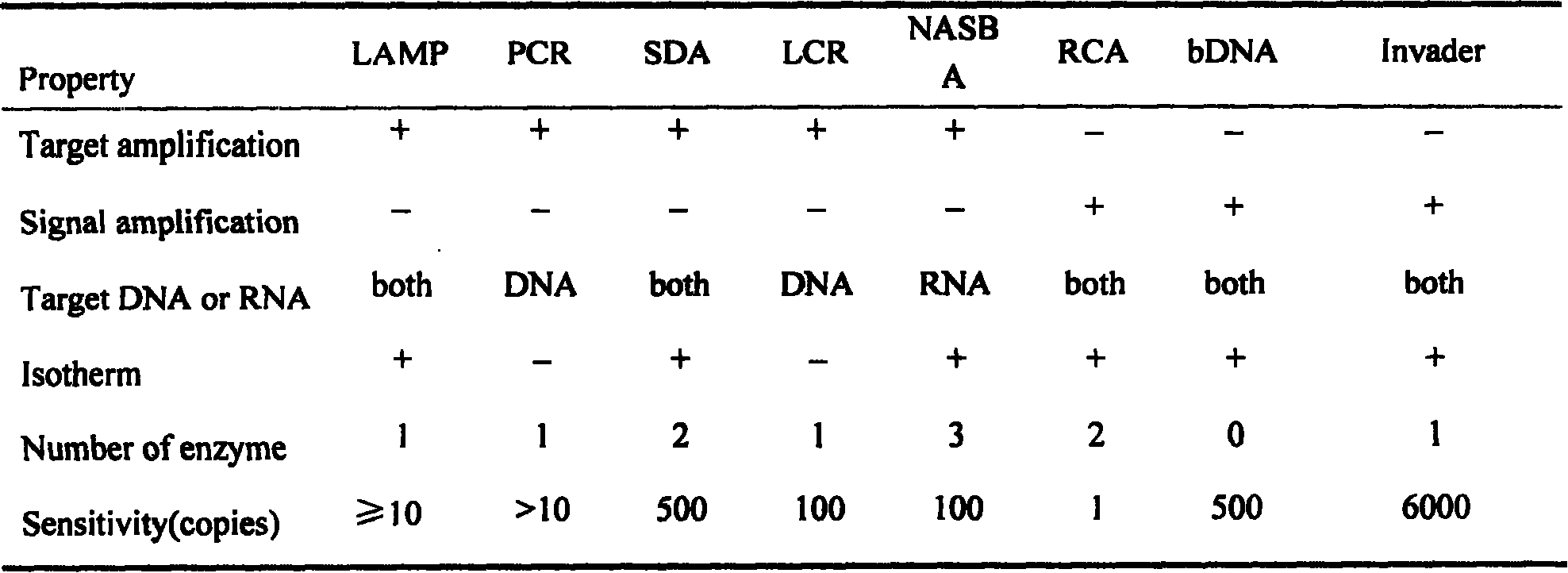
A loop-mediated isothermal, RT-LAMP technology, applied in the field of temperature amplification technology, can solve the problems of high price, high equipment requirements, RNase and nucleic acid amplification pollution, etc., to reduce pollution opportunities, facilitate quality control work, Detects the effect of improved specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
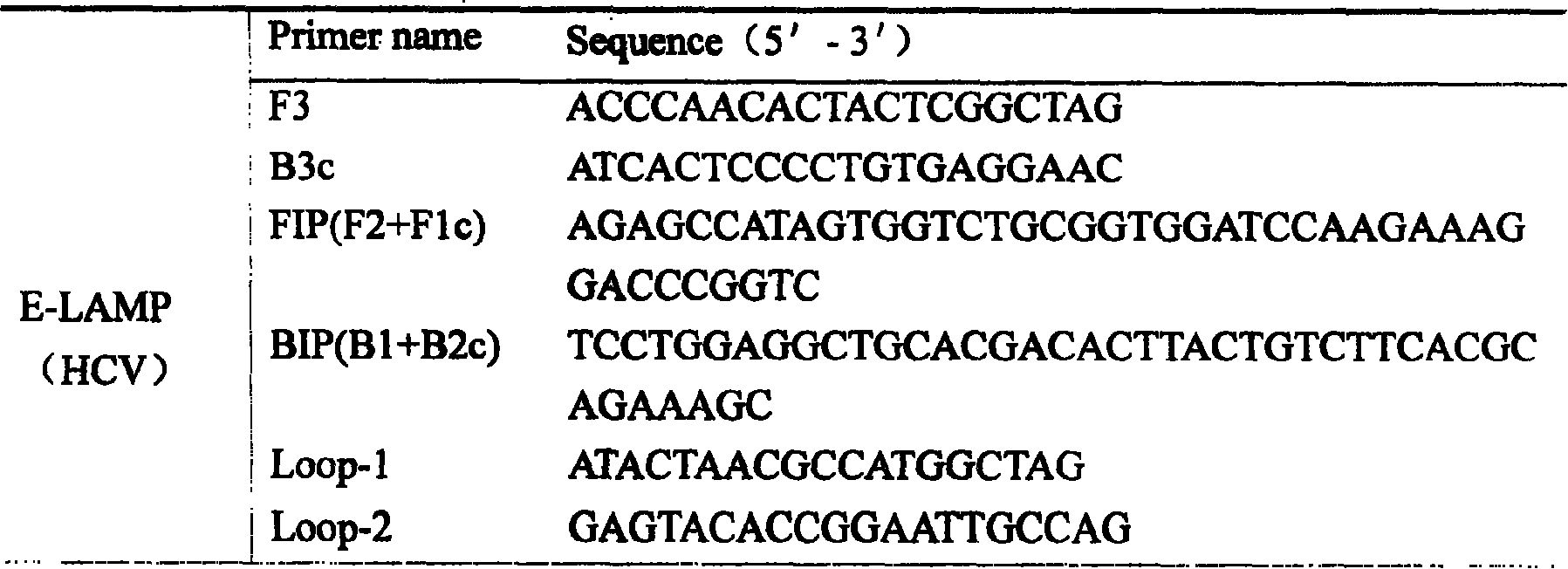
[0048] Embodiment 1: E-LAMP detection of hepatitis C virus gene (HCV)
[0049] (1) Coat streptavidin at a coating concentration of 5 μg / ml on a microplate or a microwell reaction plate, add 100 μl to each well, overnight at 4°C, pat dry the microplate, and set aside.
[0050] (2) Wash the plate with PBS, then block with 1% BSA at 37°C for 1 hour, wash the plate, and store at -20°C for later use.
[0051] (3) Add the E-LAMP reaction system with labeled primers, and amplify at 63° C. for 1.5 h.
[0052] The reaction system of E-LAMP is as follows: F3 and B3, 5pmol; BIP and FIP, 40pmol; Loop-1 and Loop-2, 20pmol; the final concentration of other reaction components is 1.0mM dNTP, 1mM betaine, 6mMMgSO4, 2.5μl 10× Bst-DNA Polymerase Buffer, 8U Bst-DNA polymerase, 1U AMV reverse transcriptase, 5 μl HCV RNA sample, replenish water to 25 μl, mix well, 63°C, water bath for 1.5h.
[0053] (4) Remove the reaction solution in the well and wash the plate.
[0054] (5) Add HRP-labeled an...
Embodiment approach 2
[0058] Implementation Option 2: Sensitivity Test for E-LAMP Solution
[0059] In order to verify the semi-quantitative effect of E-LAMP and the feasibility of routine gene detection, a 10-fold serial dilution was performed on a quantified HCV RNA sample, and the sensitivity of the E-LAMP solution was tested using the diluted sample. The specific operating procedures were as follows: The E-LAMP detection step is performed. The test results show that the sensitivity of this method can theoretically detect 10 copies of HCV cDNA molecules, which is the same as that of ordinary RT-LAMP, which proves that the sensitivity of this technology has not been affected after the modification.
Embodiment approach 3
[0060] Embodiment 3: E-LAMP detects the specificity of HCV virus
[0061] The method is the same as the E-LAMP detection of hepatitis C virus gene (HCV), and the RNA samples used are changed to HIV and HBV. The experimental results show that the OD values of HIV and HBV tend to be negative, which proves that the specificity of this detection method is high.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com