Chinese medicine and mass control method of preparations thereof
A quality control method and preparation technology, which is applied in the direction of pharmaceutical formulations, measuring devices, drug combinations, etc., can solve the problems of drug safety, effectiveness, low ursolic acid content, low extraction rate of fat-soluble components, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
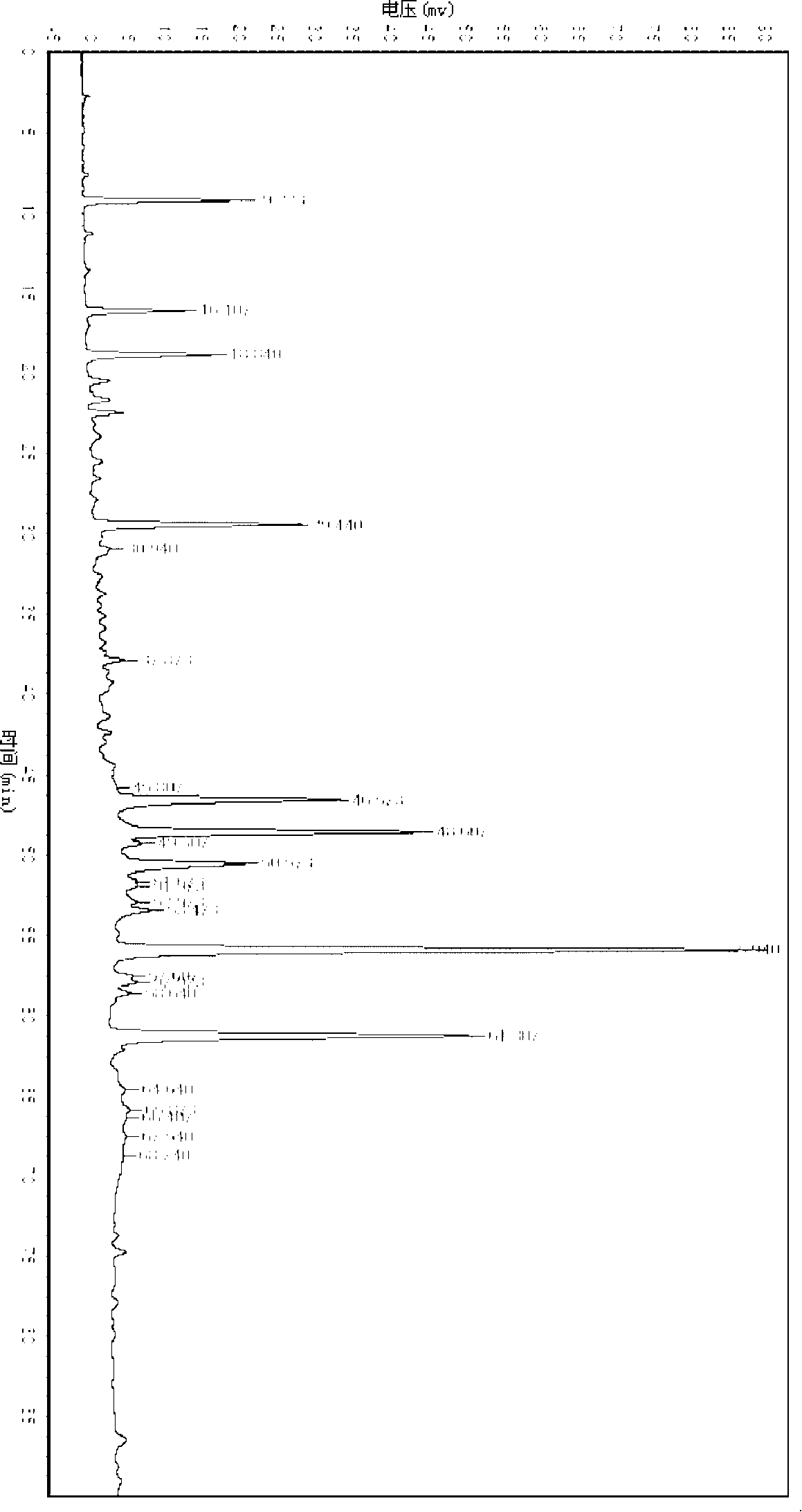
[0068] Example 1: Add 1 g of Shanxiang round leaf powder or an appropriate amount of preparation (such as 1 g of Shanxiangyuan sugar-reducing granules, 1 piece of Shanxiang round tablet or a preparation approximately equivalent to 1 g of medicinal materials), add petroleum ether for Soxhlet extraction for 1 hour, discard the petroleum ether, The petroleum ether was evaporated from the residue, an appropriate amount of methanol was added, refluxed for extraction for 1 hour, the methanol solution was taken, concentrated to 2ml, and used as the test solution. In addition, get gallic acid reference substance, add methanol to make a solution containing 1mg per 1ml, as a reference substance solution, test according to thin-layer chromatography ("Chinese Pharmacopoeia" 2005 edition one appendix VIB) test, draw the above-mentioned need testing solution, contrast Each 2μl of the solution of the product was spotted on the same polyamide film, developed with 36% acetic acid as the develop...
Embodiment 2
[0069]Example 2: 1g of the powder of the round leaves of Shanxiang or an appropriate amount of preparation (such as 1g of the sugar-reducing granules of Shanxiangyuan, 1 tablet of Shanxiangyuan or a preparation equivalent to 1g of medicinal materials), add 30ml of water, reflux for 1 hour, filter, combine the filtrates, and concentrate the filtrates to 10ml, adjust the pH value to 3-4 with dilute hydrochloric acid, extract 4 times with water-saturated n-butanol, 10ml each time, combine the n-butanol solution, recover the n-butanol solution, add 1ml methanol to the residue to dissolve, and use it as the test solution . Get gallic acid reference substance in addition, add methanol and make every 1ml solution containing 1mg, as reference substance solution, test according to thin-layer chromatography ("Chinese Pharmacopoeia" 2005 edition one appendix VI B) test, draw above-mentioned need testing solution, Each 2 μl of the reference solution was spotted on the same silica gel G th...
Embodiment 3
[0070] Embodiment 3: test according to thin-layer chromatography ("Chinese Pharmacopoeia" 2005 edition one appendix VI B), draw each 2 μ l of need testing solution and reference substance solution under the item of embodiment 2, respectively spot on the same silica gel GF254 thin-layer plate Above, use chloroform-ethyl acetate-formic acid (6:4:1) as developing solvent, develop, take out, dry in the air, inspect under ultraviolet 254nm, in the chromatogram of the test product, at the position corresponding to the chromatogram of the reference product, a significant Spots of the same color.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com