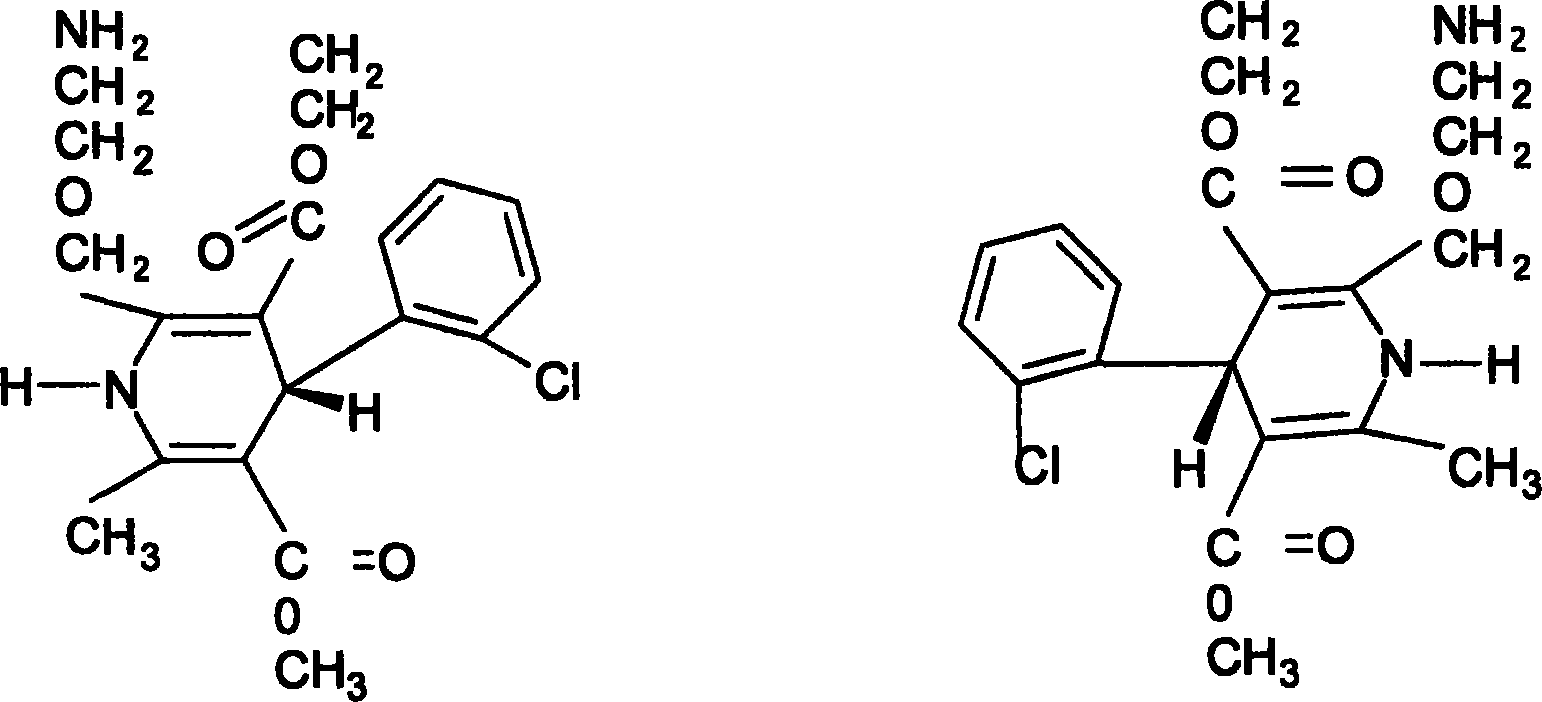
Medicament compound containing levorotatory ammonia chlorine horizon and atorvastatin and preparing method thereof
A technology of levamlodipine and atorvastatin, which is applied in the field of pharmaceutical compositions and preparations containing levamlodipine and atorvastatin, can solve the problems of poor patient compliance, complicated process, fragile tablets, etc., and achieve High bioavailability, simple preparation process, and less intestinal residue
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0065] The method for preparing the oral immediate-release preparation of the present invention is known in the prior art, and a person of ordinary skill in the art can select a suitable method and process from the prior art to prepare the present invention according to the description of the present invention. oral immediate-release formulations. It has been reported to prepare orally disintegrating tablets by freeze-drying (DilipJG. Ann Arbor, Mich. Fast dissolving solid dosage from comprising a porous network of matrix material [P]. US5,558,880 1996). In a preferred example of the present invention, the preparation method comprises directly mixing levamlodipine and its pharmaceutically acceptable salts and atorvastatin and its pharmaceutically acceptable salts and pharmaceutically acceptable salts of the present invention. acceptable carrier (such as fillers and / or adsorbents, disintegrants, wetting agents and / or binders, lubricants and / or glidants, flavoring agents, etc.),...
Embodiment 1
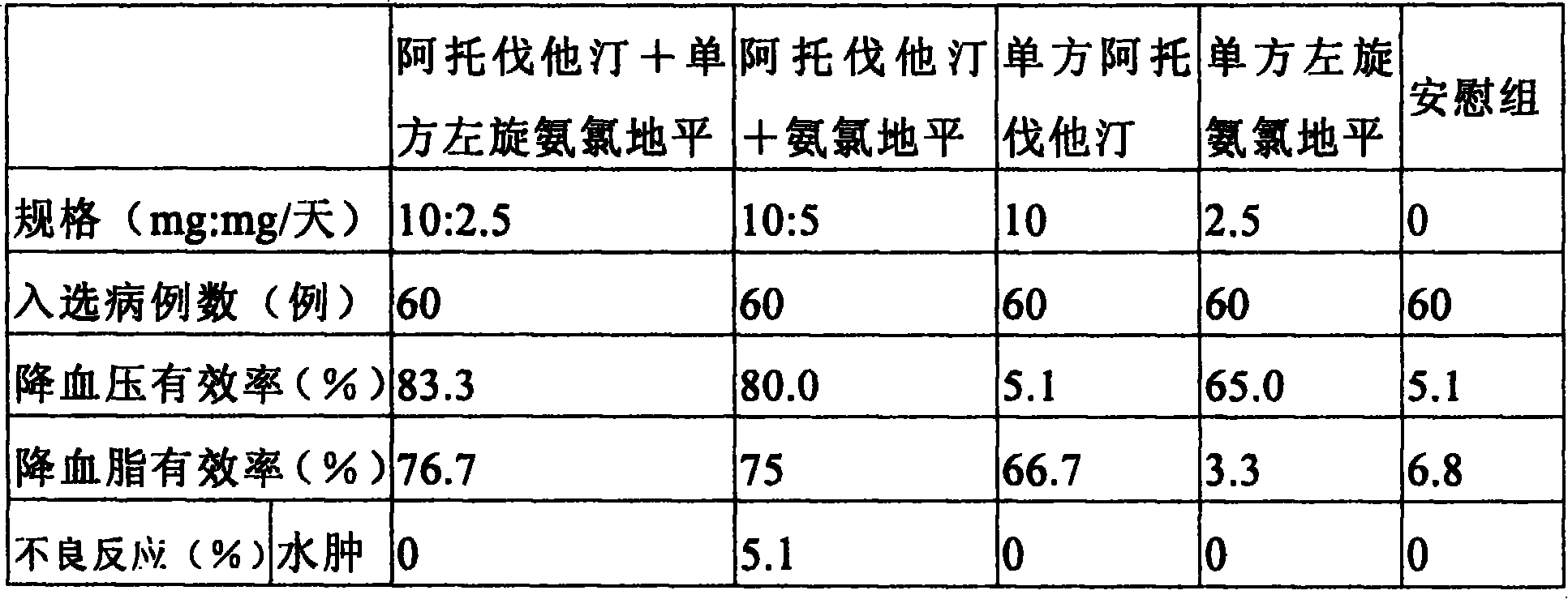
[0076] As shown in Table 1, atorvastatin (purchased from Beijing Maijin Pharmaceutical Technology Co., Ltd.) and levamlodipine (purchased from Ningbo Red Eagle Pharmaceutical Co., Ltd.) of the stated weight were mixed, and then sequentially Add microcrystalline cellulose, low-substituted hydroxypropyl cellulose, mannitol, and croscarmellose starch sodium of the weight described in Table 1 and mix evenly, and directly compress tablets (8 punching tablet machines, manufacturer RIMEK, model: MiniPRESS- 11SF), 400 oral immediate release formulations. The resulting tablets were tested and the results obtained are listed in Table 1 below.
Embodiment 2-7
[0078] Oral immediate-release formulations were prepared as described in Example 1, except that the components and amounts used were shown in Table 1 below. The resulting tablets were tested and the results obtained are listed in Table 1 below.
[0079] Table 1
[0080] implement
[0081] It can be seen from the above table that the obtained oral immediate-release preparation has good mouthfeel, short disintegration time, high hardness, and does not require special packaging, thereby achieving the purpose of the present invention.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com