Active peptide and application thereof
A technology of activity and activity inhibition, applied in the field of active single peptides, can solve the problems that the inhibitory activity of active single peptide ACE has not been reported yet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The preparation of embodiment 1 oyster soluble protein
[0024] Shuck and gut fresh oysters. The remaining white oyster meat, gills and body fluids are chopped with a homogenizer at low temperature. After that, it was added to an equal volume of PBS (0.2mol / L NaH 2 PO 4 and 0.2mol / L Na 2 HPO 4 Mix by volume ratio 39:61, pH 7.0), stir well. After standing at 4°C for 6 hours, centrifuge (5000×g, 4°C) for 25 minutes. The supernatant was taken, ammonium sulfate was added to make the final concentration reach 75%, and it was left standing for 6 hours at 4°C, and then centrifuged (5000×g, 4°C) for 25 minutes. The precipitate was mixed with a small amount of water and loaded into a dialysis bag (MW 10000). Dialyze with running water for 24 hours, and then with deionized water for 24 hours, then vacuum freeze-dry the content to obtain oyster crude protein powder, and store at -20°C.
Embodiment 2
[0025] The enzymolysis of embodiment 2 oyster soluble protein
[0026] Oyster soluble protein was dissolved in 0.05 mol of phosphate buffer (adjusted to pH 2.0 with 1 mol / L HCl), the protein concentration was 2.5%, the ratio of enzyme to substrate was added to pepsin, and the ratio of enzyme to substrate was 1 :50 (mass ratio), enzymatic hydrolysis at 37°C for 24 hours. The obtained enzymatic hydrolysis solution was heated to 100°C and kept for 10 minutes to inactivate the protease. Then adjust the pH of the hydrolyzate to be neutral, centrifuge (5000 × g, room temperature) for 25 minutes, filter through an ultrafiltration membrane (MW 10000), and vacuum freeze-dry the components that pass through the ultrafiltration membrane to obtain oyster oligopeptides,- Store at 20°C.
Embodiment 3
[0027] The separation of embodiment 3 oyster oligopeptide
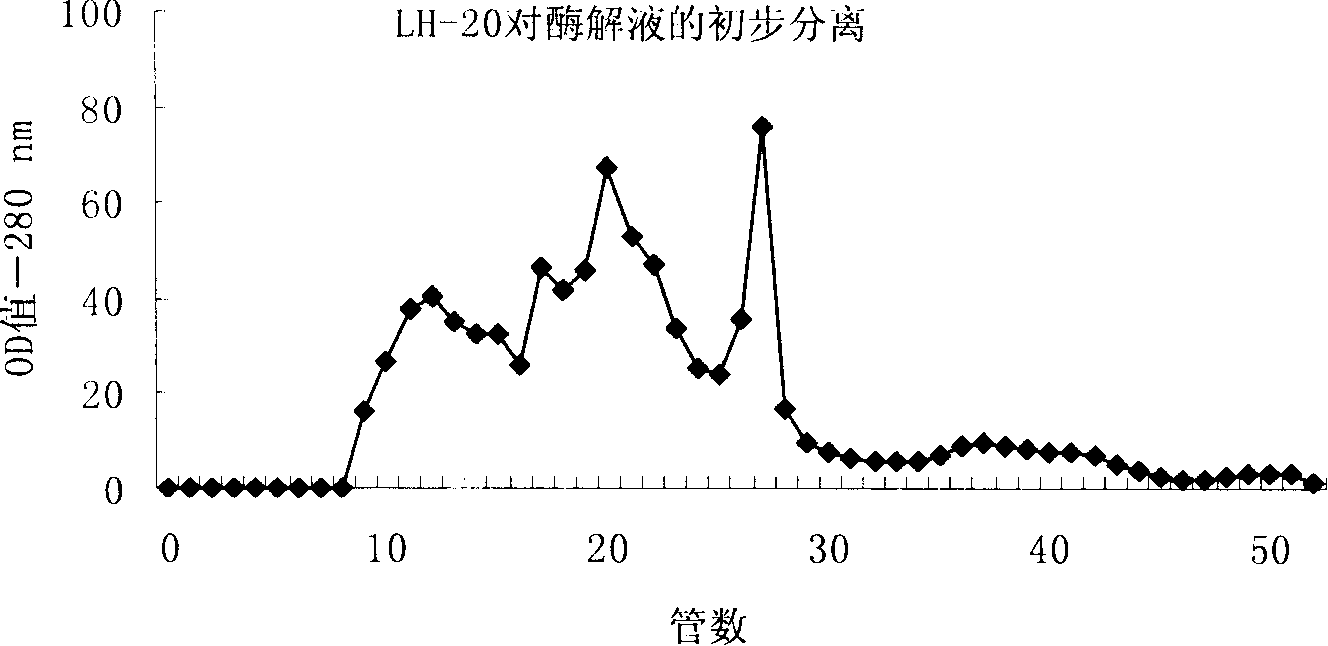
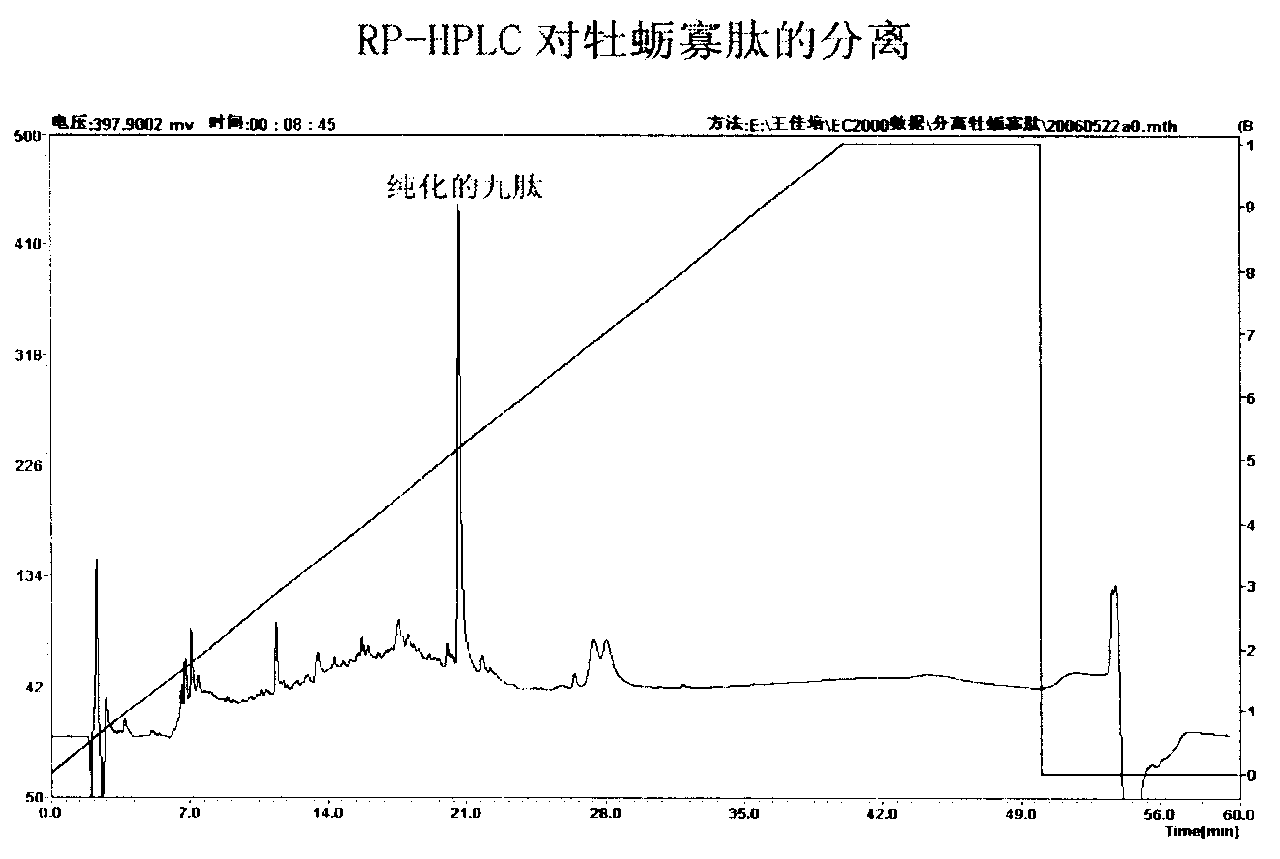
[0028] Carry out chromatographic separation (such as figure 1 shown), elute with 30% methanol solution, collect according to the number of tubes, 20 minutes / tube, collect the part with strong ACE inhibitory activity and continue to separate and purify on RP-HPLC (such as figure 2 shown),. Hypersil C on RP-HPLC 18 Column, mobile phase A: 0.1% TFA aqueous solution, ultrasonic degassing before use; mobile phase B: 100% acetonitrile, detection wavelength 215nm, room temperature, injection volume 20μl, gradient elution, collected according to time .
[0029] Elution gradient: 0-40min A solution 100%-0% linear decrease; B solution 0%-100% linear increase, 40-50min A solution 0%; B solution 100%.
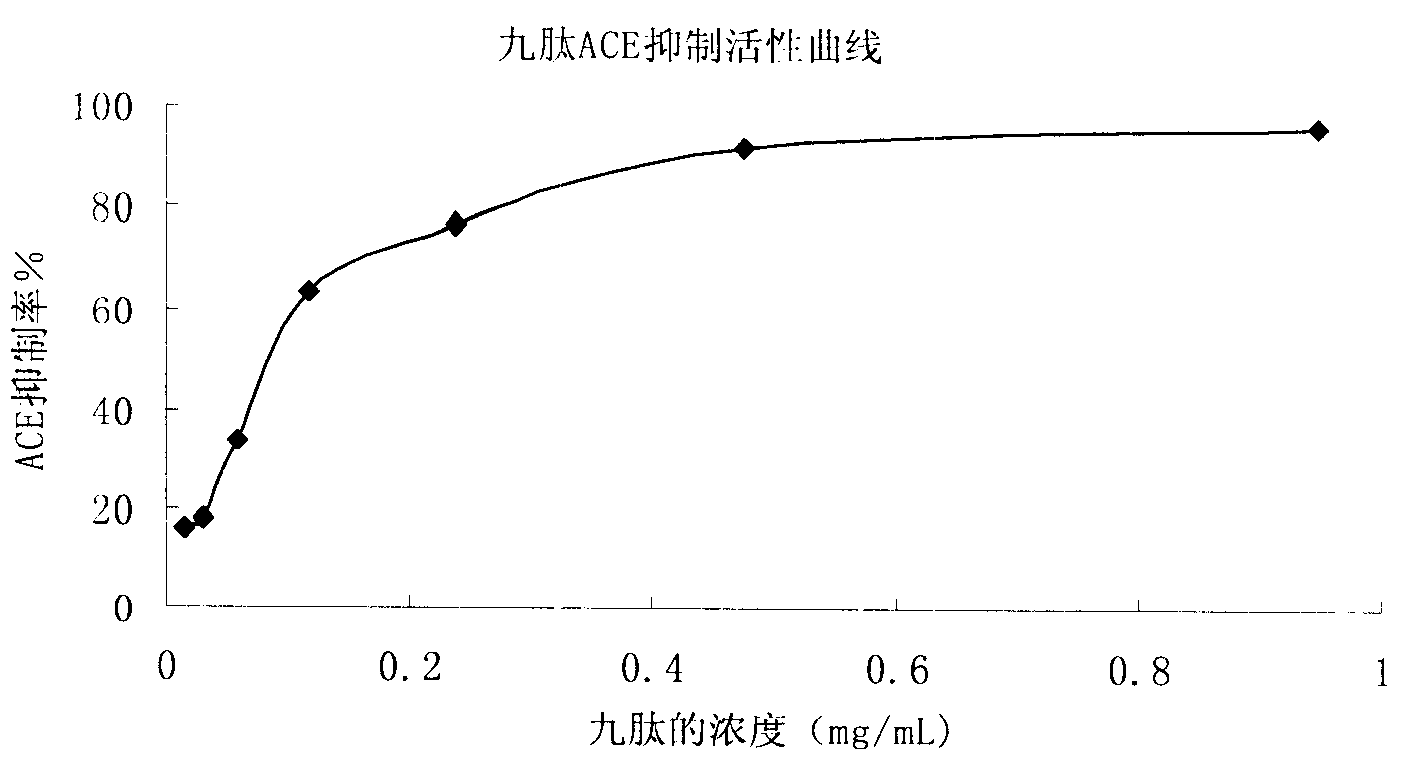
[0030] The obtained active single peptide has the amino acid sequence of NO.1 in the sequence table.
[0031] The molecular weight of this active peptide is determined to be 1195 Daltons, and its amino acid sequence is valin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com