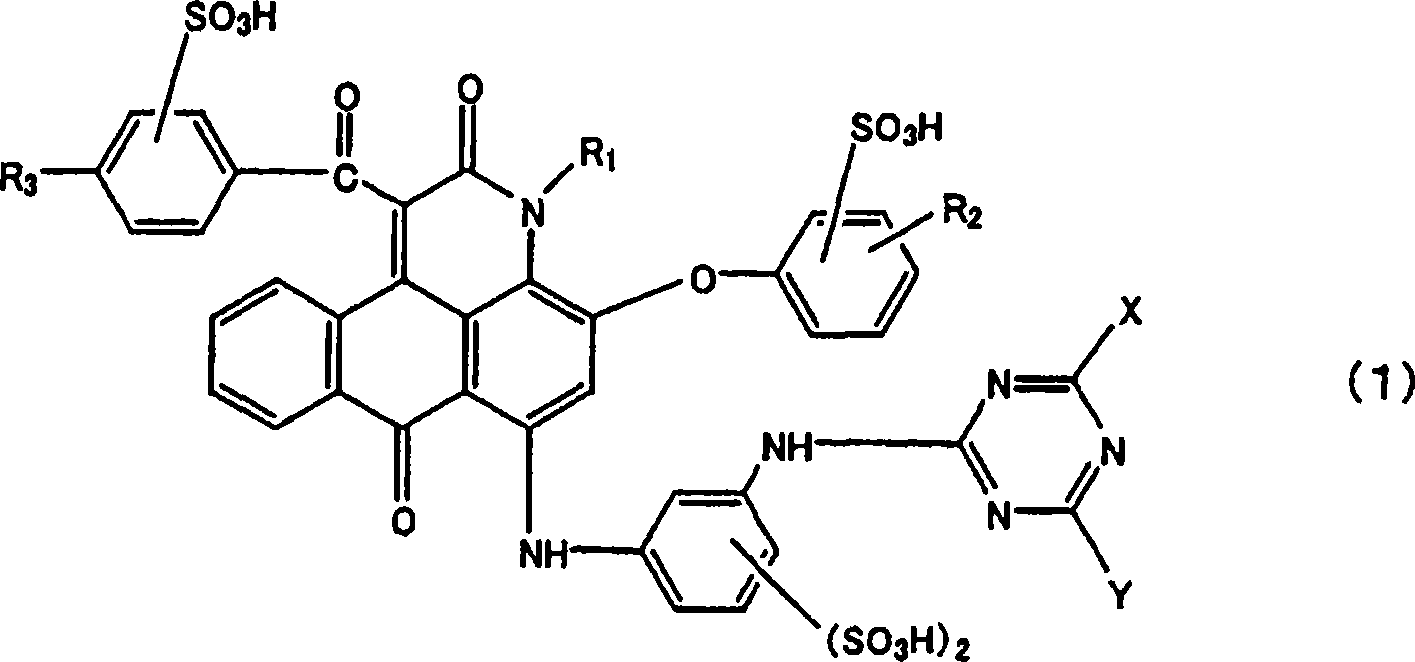
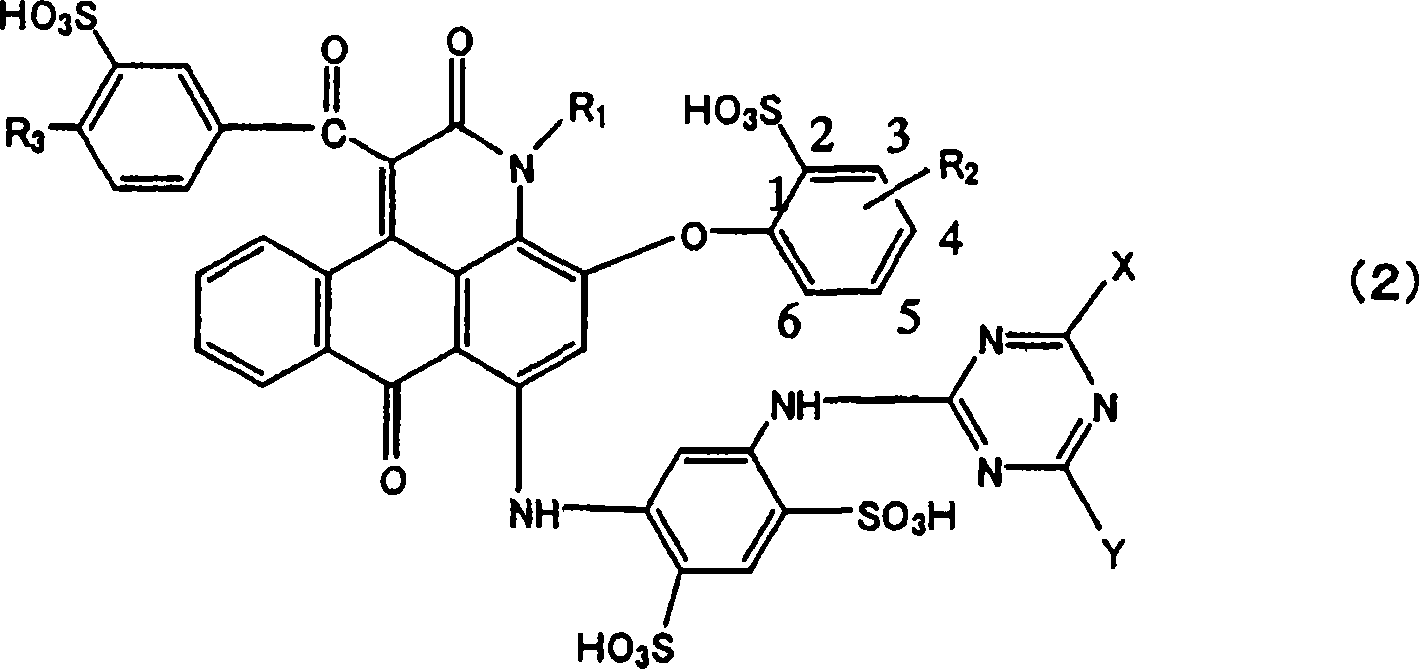
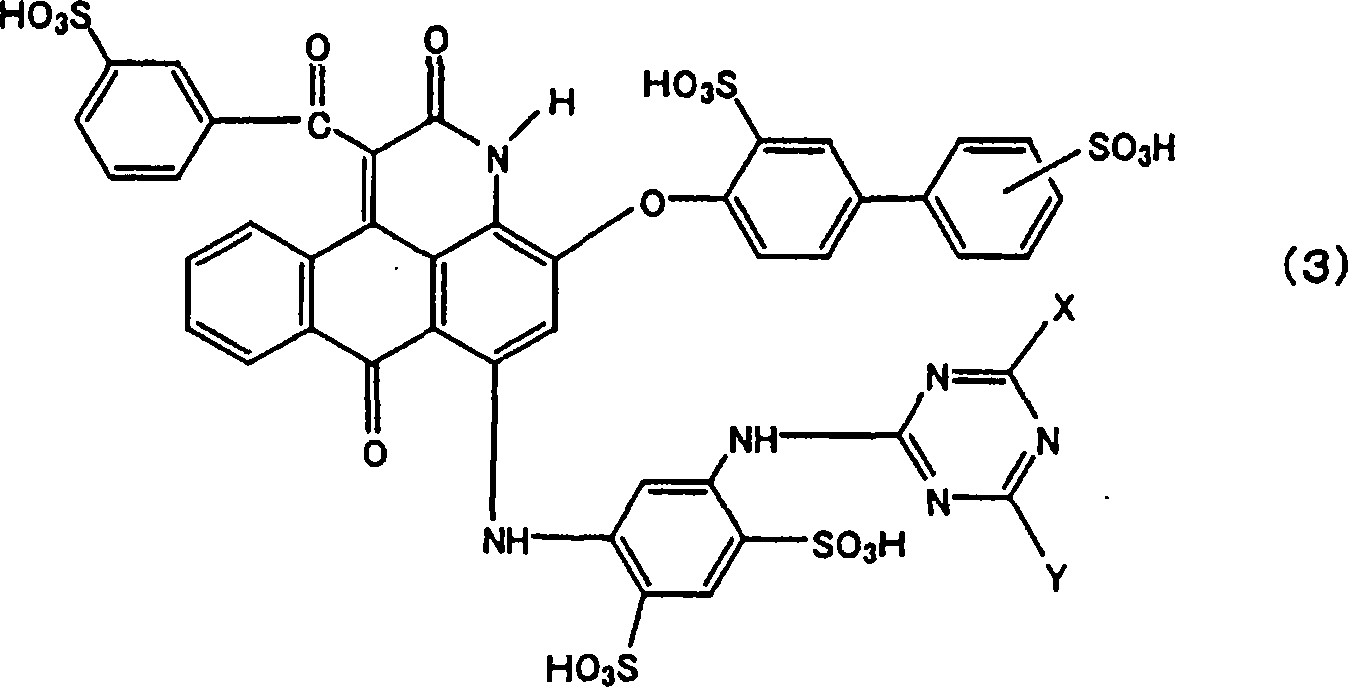
Anthrapyridone compound and salt thereof, magenta ink composition containing such anthrapyridone compound, and colored body
An anthrapyridone and compound technology, applied in the field of magenta ink composition, can solve the problems of light fastness, gas resistance, poor sharpness, poor light fastness, etc., and achieve good fastness, good filterability, good storage stability sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0156] (1) To 375.0 parts of N,N-dimethylformamide, while stirring, add the compound (R 1 =H) 381.0 parts, the compound (R 2 =4-phenyl)(4-hydroxybiphenyl) 44.6 parts, and 19.0 parts of pulverized (100 mesh) potassium carbonate, and raised temperature, reacted at 130 to 140° C. for 3 hours. The obtained reaction solution was cooled with water, 450 parts of methanol was added thereto, and after stirring for 30 minutes, the reaction product was filtered, separated, washed successively with 700 parts of methanol and 300 parts of warm water at about 80°C, and dried to obtain 65.9 parts of the above The compound of formula (12) (R 1 = H, R 2 =4-phenyl) as yellow-brown crystals.
[0157] (2) To 120 parts of xylene, while stirring, add 65.8 parts of the compound (R 1 = H, R 2 =4-phenyl), 1.4 parts of potassium carbonate, 67.2 parts of ethyl benzoylacetate (compound of formula (13), R 3 =H), and raise the temperature. The reaction was carried out at a temperature of 142 to 144° ...
Embodiment 2
[0175](1) The reaction solution containing the compound represented by the formula (17) was obtained by performing the operations of (1) to (5) in Example 1. Add an aqueous solution consisting of 3.2 parts of anthranilic acid, 3.7 parts of 25% caustic soda aqueous solution and 15 parts of water to the reaction solution, and add 25% caustic soda dropwise at a temperature of 27 to 30°C. Aqueous solution, while keeping the pH at 4.8 to 5.2, carry out the secondary condensation reaction for 30 minutes to obtain the reaction solution containing the compound represented by formula (19).
[0176]
[0177] (2) Add an appropriate amount of 25% caustic soda aqueous solution to the reaction liquid containing the compound of formula (19) obtained in the operation of the above (1), while maintaining the pH at 10.8 to 11.2, and at a temperature of 90 to 95° C. The reaction was carried out for 2 hours. After the reaction, water was added to the reaction liquid to adjust the liquid volume...
Embodiment 3
[0182] (1) The reaction solution containing the compound represented by the formula (17) was obtained by performing the operations of (1) to (5) in Example 1. To this reaction solution, add an aqueous solution consisting of 4.4 parts of 5-aminoisophthalic acid, 7.7 parts of 25% caustic soda aqueous solution and 15 parts of water, and drop 25% of Aqueous caustic soda solution, while maintaining the pH at 5.8 to 6.2, carried out the secondary condensation reaction for 30 minutes to obtain a reaction solution containing the compound shown in the following formula (20).
[0183]
[0184] (2) Add an appropriate amount of 25% caustic soda aqueous solution to the reaction liquid containing the compound of formula (20) obtained in the operation of the above (1), while maintaining the pH at 10.8 to 11.2, and at a temperature of 90 to 95° C. React for 2 hours. After the reaction, water was added to the reaction liquid to adjust the liquid volume to 250 parts, and the insoluble matte...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorption wavelength | aaaaa | aaaaa |
| absorption wavelength | aaaaa | aaaaa |
| absorption wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com