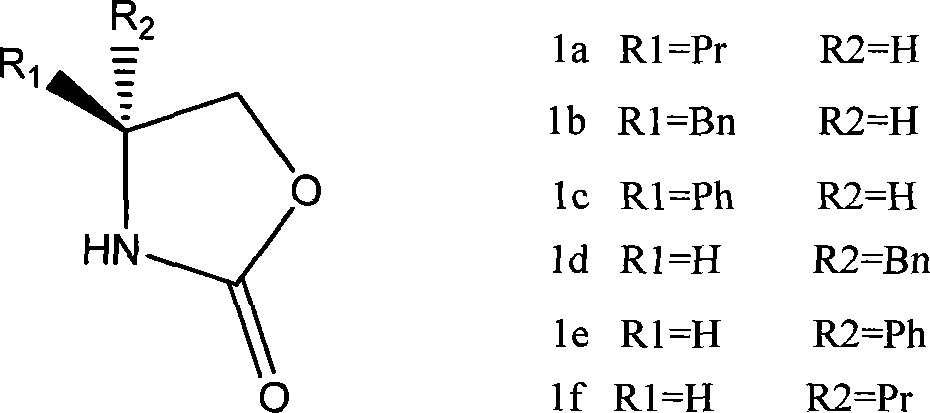Method for preparing 4-substituted chirality oxazolidinone compounds
A technology for oxazolidinones and compounds, applied in the field of preparation of chiral 4-substituted oxazolidinones, can solve problems such as potential safety hazards, and achieve the effects of reducing waste water discharge, reducing production costs, and reducing potential safety hazards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
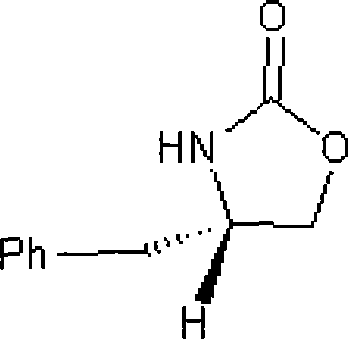
[0019] Example 1: Preparation of s-(+)-4-benzyl-2-oxazolidinone
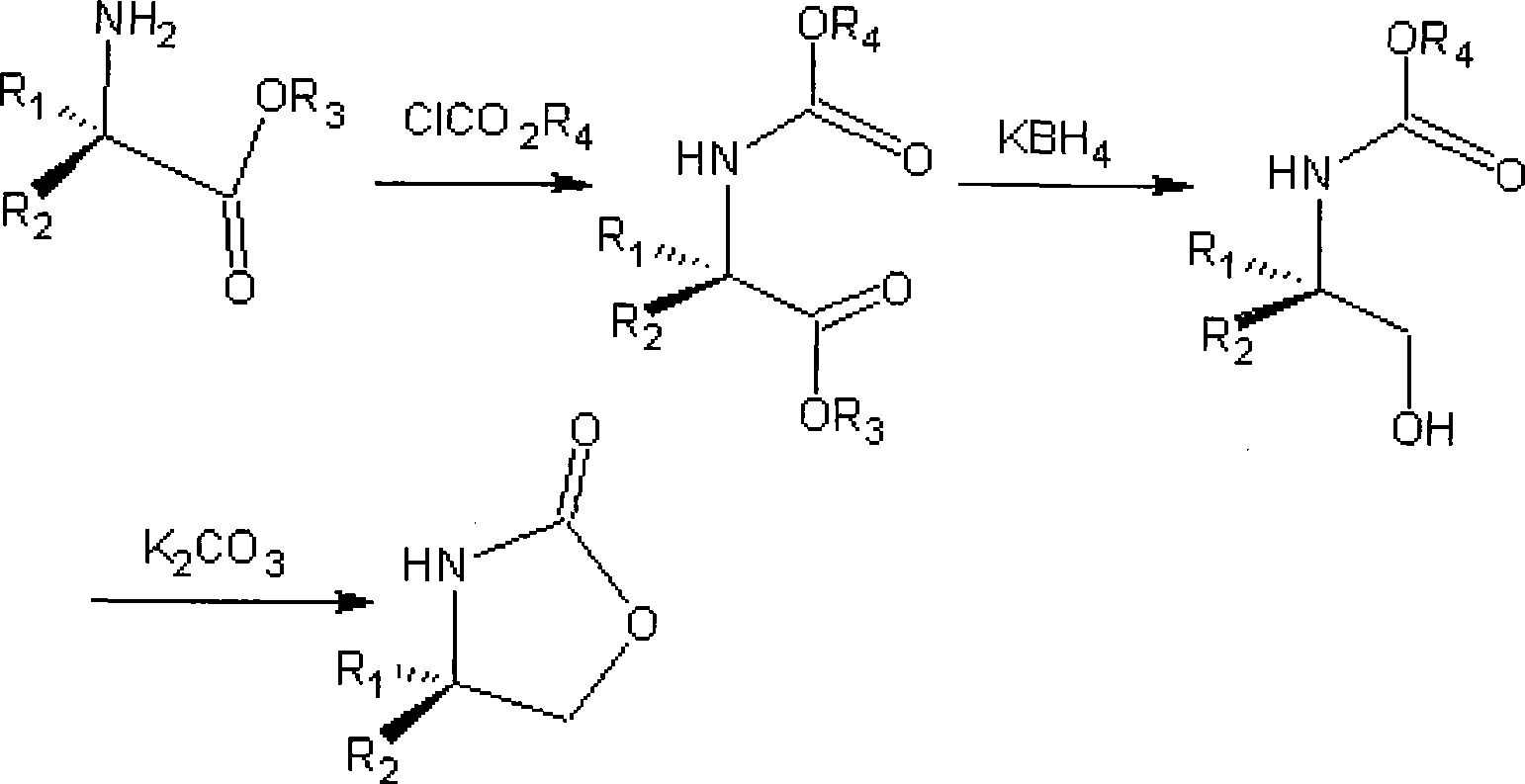
[0020] Add 26.1 g (0.12 mol) of L-phenylalanine methyl ester hydrochloride to 100 ml of dichloromethane, 30.2 g (0.36 mol) of sodium bicarbonate, and dropwise add 14.3 g (0.13 mol) of methyl chloroformate at room temperature, After dripping, stir at room temperature for 6 hours, filter, recover the solvent dichloromethane, and obtain an oil Add 400ml of absolute ethanol, add 13.3g (0.12mol) of calcium chloride, 12.9g (0.24mol) of potassium borohydride, react at 15-25°C until the reduction is complete, recover the solvent ethanol, add 150ml of 1M citric acid, and use ethyl acetate The ester was extracted twice, each time the amount of extraction was 200ml, the organic layers were combined, and the solvent ethyl acetate was recovered to obtain the reduced product Add 160ml of toluene, 33g (0.24mol) of potassium carbonate, heat to reflux to separate water for 2 hours, suction filter while it is hot, cool and cry...
example 2
[0023] Example 2: Preparation of R-(-)-4-benzyl-2-oxazolidinone
[0024] D-phenylalanine methyl ester hydrochloride 26.1g (0.12mol) was added dichloromethane 100ml, sodium bicarbonate 30.2g (0.36mol), at room temperature was added dropwise ethyl chloroformate 14.3 grams (0.13mol), After dripping, stir at room temperature for 6 hours, filter, recover the solvent dichloromethane, and obtain an oil , add 400ml of absolute ethanol, add 13.3g (0.12mol) of anhydrous calcium chloride, 12.9g (0.24mol) of potassium borohydride, react at 15-25°C until the reduction is complete, recover the solvent ethanol, add 150ml of 1M citric acid, Two extractions were carried out with ethyl acetate, each time the amount of extraction was 200ml, the organic layers were combined, and the solvent ethyl acetate was recovered to obtain the reduced product. Add 160ml of toluene, 33g (0.24mol) of potassium carbonate, heat to reflux to separate water for 2 hours, suction filter while hot, crystallize the ...
example 3
[0027] Example 3: Preparation of s-(+)-4-phenyl-2-oxazolidinone
[0028] Add 100 ml of methylene chloride and 30.2 g (0.36 mol) of sodium bicarbonate to 24.4 g (0.12 mol) of L-phenylglycine methyl ester hydrochloride, add 14.3 g (0.13 mol) of ethyl chloroformate dropwise at room temperature, drop Stir at room temperature for 6 hours, filter, and recover the solvent dichloromethane to obtain off-white solid Add 400ml of absolute ethanol, add 13.3g (0.12mol) of anhydrous calcium chloride, 12.9g (0.24mol) of potassium borohydride, react at 15-25°C until the reduction is complete, recover the solvent ethanol, add 150ml of 1M citric acid, and use Ethyl acetate was extracted twice, each extraction dosage was 200ml, the organic layers were combined, and the solvent ethyl acetate was recovered to obtain the reduced product Add toluene 160ml, potassium carbonate 33g (0.24mol), heat to reflux for 2 hours, filter while hot, the filtrate is cooled and crystallized, suction filtered, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com