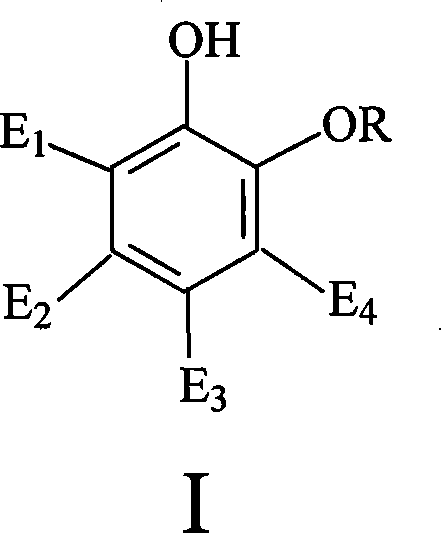
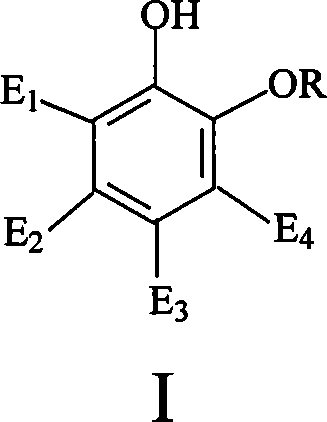
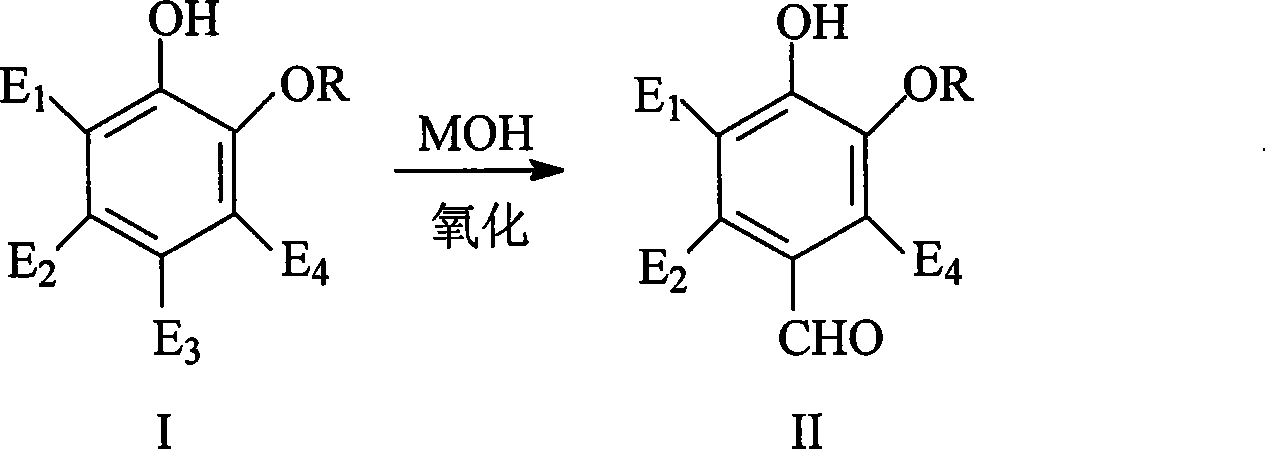
Catalyst for synthesizing vanillin and derivative and preparation
A catalyst and derivative technology, applied in the field of supported catalysts and preparation, can solve the problems of difficult source of glyoxylic acid raw materials, complicated separation process, low reaction efficiency, etc., and achieve strong industrial application value, high product purity, and high selectivity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Weigh 1.20g Co(NO 3 ) 2 ·6H 2 O, 1.30g Cu(NO 3 ) 2 ·3H 2 O, 0.05g Fe(NO 3 ) 3 9H 2 O, 0.02gCe(NO 3 ) 3 ·6H 2 O Add water to a beaker to make 12mL impregnating liquid, mix 10g γ-Al 2 o 3 The carrier was added to the impregnating solution, stirred evenly, and after standing still for 24 hours, it was dried at 120°C, calcined in a muffle furnace at 600°C for 2 hours, and after cooling, it was finely ground to obtain Catalyst 1#, which was evaluated using Scheme 1, with air as the oxidant. The reaction results are shown in Table 1.
Embodiment 2
[0032] Weigh 3.69g Co(NO 3 ) 2 ·6H 2 O, 2.75g Cu(NO 3 ) 2 ·3H 2 O, 0.41g Fe(NO 3 ) 3 9H 2 O, 0.09gMn(CH 3 COO) 2 4H 2 O, 0.257g Ni(NO 3 ) 2 ·6H2 O, 0.020gCe(NO 3 ) 3 ·6H 2 O, add water to a beaker to make 12mL impregnation solution, put 10g TiO 2 The carrier was added to the impregnating solution, stirred evenly, and after standing still for 24 hours, it was dried at 120°C, roasted in a muffle furnace at 600°C for 2 hours, and after cooling, it was pulverized to obtain Catalyst 2#, which was evaluated using Scheme 1, with air as the oxidant. The reaction results are shown in Table 1.
Embodiment 3
[0034] Weigh 1.4g Co(NO 3 ) 2 ·6H 2 O, 1.89g Cu(NO 3 ) 2 ·3H 2 O, 0.02g Fe(NO 3 ) 3 9H 2 O, 0.02gMn(CH 3 COO) 2 4H 2 O, 0.068g Zn(NO 3 ) 2 ·6H 2 O, 0.13g Ce(NO 3 ) 3 ·6H 2 O was prepared in a beaker to make 12mL impregnating solution, and 5g γ-Al 2 o 3 and 5g TiO 2 The composite carrier was added into the impregnating solution, stirred evenly, and after standing still for 24 hours, it was dried at 120°C, roasted in a muffle furnace at 600°C for 3 hours, and after cooling, it was finely ground to obtain Catalyst 3#, which was evaluated using Scheme 1, with air as the oxidant. The reaction results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com