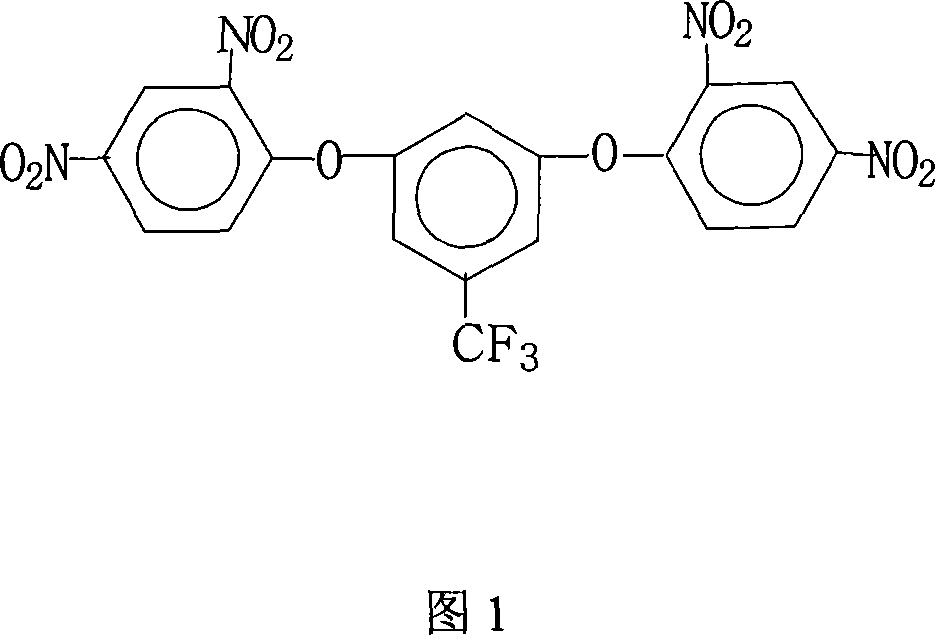
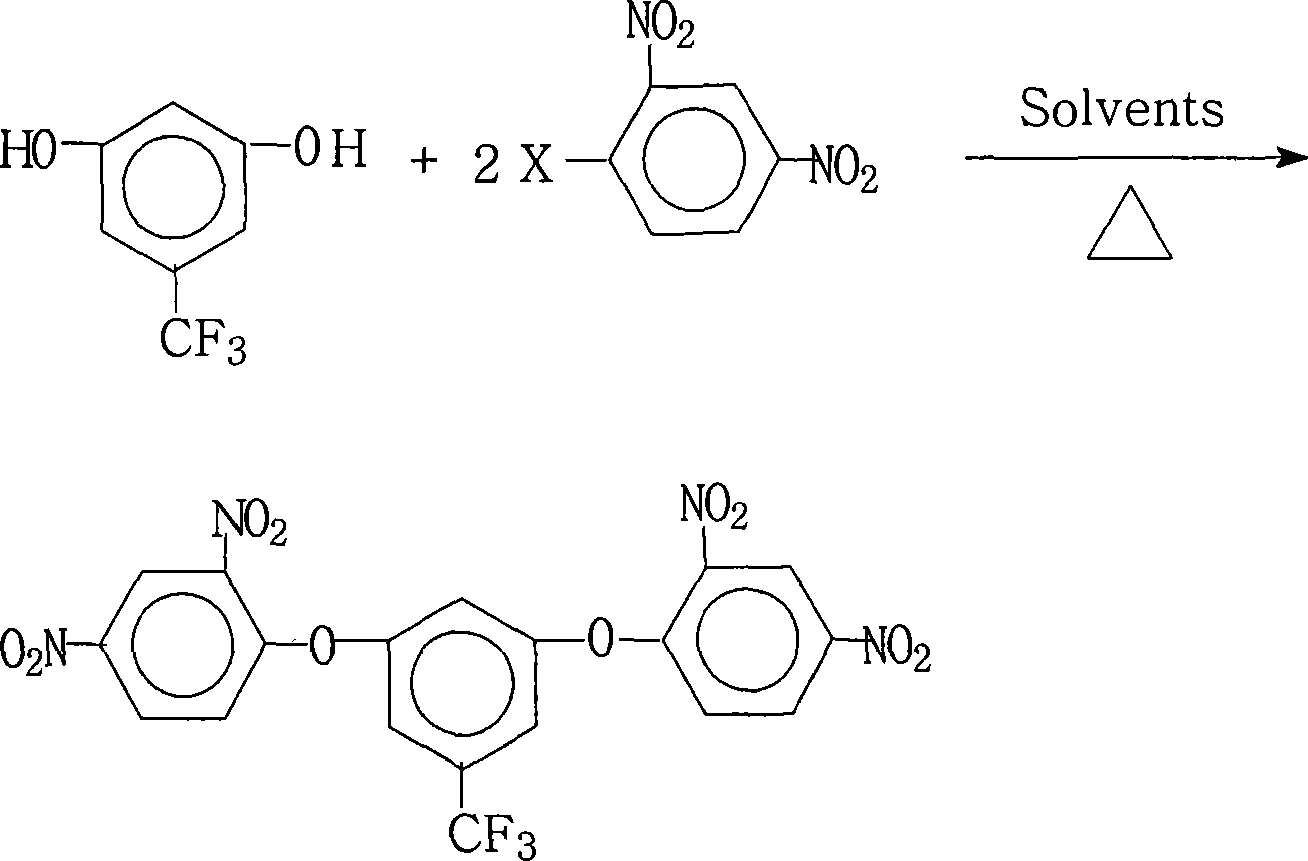
Method for preparing 3,5-di(2,4-dinitrophenoxy)trifluorotoluene
A technology of dinitrophenoxy and hydroxytrifluorotoluene is applied in 3 fields, can solve the problems such as no patents, literature reports and the like in the preparation method, and achieves the advantages of easy popularization and application, high product yield and purity, and less three wastes. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 17.8 grams (0.10 moles) of 3,5-dihydroxybenzotrifluoride, 44.6 grams (0.22 moles) of 2,4-dinitrochlorobenzene, 110.4 grams (0.80 moles) of potassium carbonate, 700 milliliters of N, N-di Methylformamide and 180 ml of toluene were put into the reaction kettle, stirred, heated to reflux and separated from water for 18 hours, then concentrated the reaction solution, recovered the solvent for recycling, cooled the reactant system, added water, precipitated a solid product, washed with hot water 2~3 times, dry, obtain 48.6 grams of 3,5-bis(2,4-dinitrophenoxy)benzotrifluorotoluene crystal product, the purity is 99.0%, obtain 3,5-bis(2, The amount and theoretical amount (51.0 grams) of 4-dinitrophenoxy)benzotrifluoride, the calculated yield of 3,5-bis(2,4-dinitrophenoxy)benzotrifluoride is 95.3% .
Embodiment 2
[0029] 17.8 grams (0.10 moles) of 3,5-dihydroxytrifluorotoluene, 54.4 grams (0.22 moles) of 2,4-dinitrobromobenzene, 55.2 grams (0.40 moles) of potassium carbonate, 150 milliliters of N, N-two Methyl acetamide and 15 milliliters of xylene were put into the reaction kettle, stirred, heated and refluxed for water separation for 3 hours, concentrated the reaction solution, recovered the solvent for recycling, cooled the reactant system, added water, and precipitated a solid product, washed with hot water Washed 2 to 3 times and dried to obtain 31.8 grams of 3,5-bis(2,4-dinitrophenoxy)trifluorotoluene crystal product with a purity of 99.1%. According to actual conditions, 3,5-bis(2, The amount and theoretical amount (51.0 grams) of 4-dinitrophenoxy)benzotrifluoride, the calculated yield of 3,5-bis(2,4-dinitrophenoxy)benzotrifluoride is 62.3% .
Embodiment 3
[0031] 17.8 grams (0.10 moles) of 3,5-dihydroxybenzotrifluoride, 40.5 grams (0.20 moles) of 2,4-dinitrochlorobenzene, 10.6 grams (0.10 moles) of sodium carbonate, 96 milliliters of N-methyl- 2-pyrrolidone and 36 milliliters of dichlorobenzene were put into the reaction kettle, stirred, heated to reflux and separated from water for 16 hours, the reaction solution was concentrated, the solvent was recovered for recycling, the reactant system was cooled, water was added, and a solid product was precipitated, and heated Washed with water for 2 to 3 times, dried to obtain 41.5 grams of 3,5-bis(2,4-dinitrophenoxy)benzotrifluorotoluene crystal product with a purity of 99.3%. According to the actual obtained 3,5-bis( 2, the amount of 4-dinitrophenoxy) trifluorotoluene and theoretical output (51.0 grams), calculate the yield of 3,5-two (2,4-dinitrophenoxy) trifluorotoluene as 81.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com