Method for preparing benzimidazole
A technology of benzimidazole and phenylenediamine, applied in the field of preparation of benzimidazole, can solve problems such as long reaction time, and achieve the effects of rapid synthesis, easy access to equipment, and convenient production scale-up
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
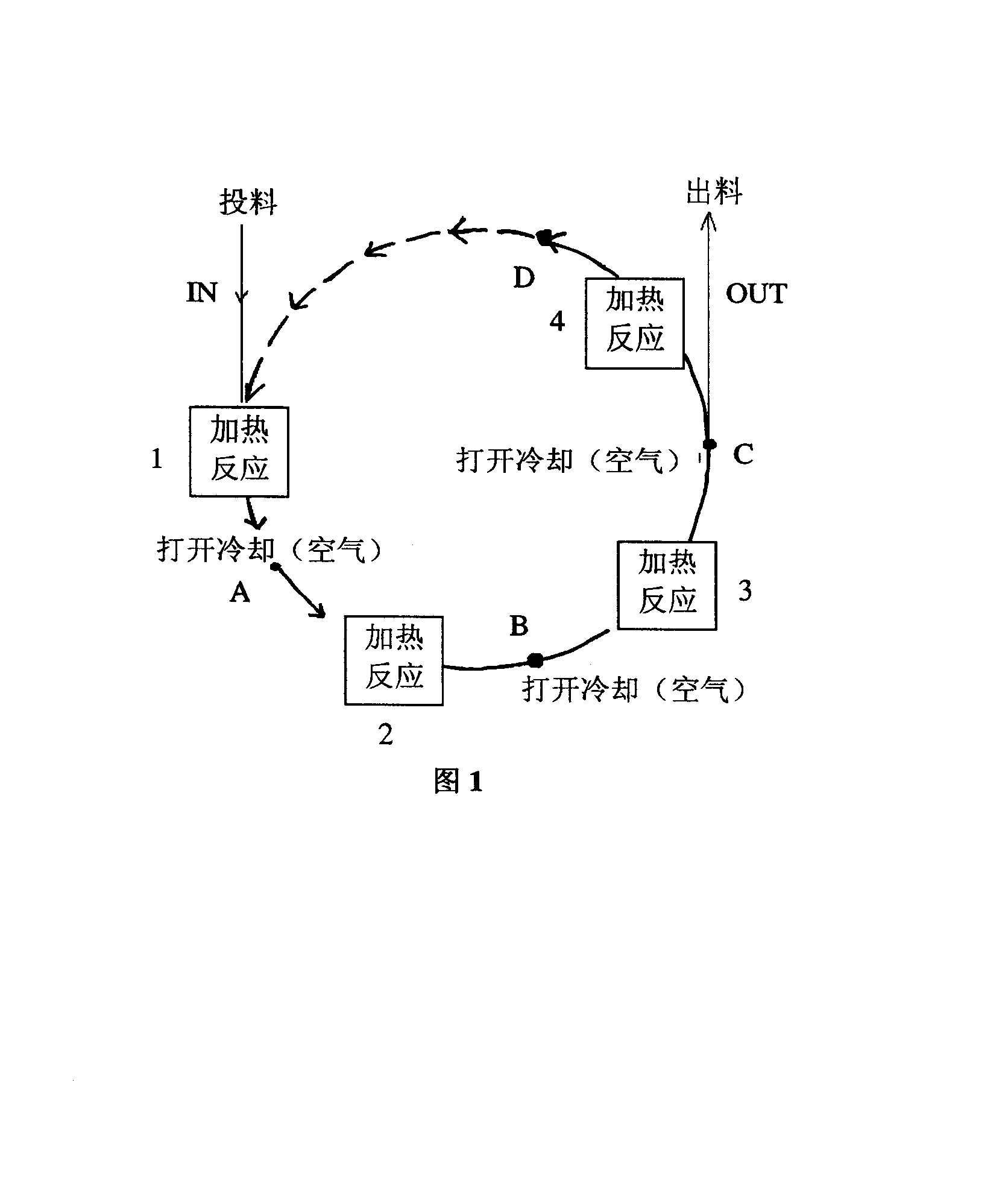
[0035] Add 0.810 g (7.5 mmol) of o-phenylenediamine, 0.954 g (9 mmol) of benzaldehyde, 0.162 g of potassium iodide and 0.75 mL of DMF into the Erlenmeyer flask in sequence. Microwave was used to heat indirectly 12 times, each time for 40 seconds. After heating, the Erlenmeyer flask was placed in the air, and the heating was continued after the temperature of the reaction mixture dropped. After the reaction was complete, the resulting mixture was dissolved in ethyl acetate, and then saturated NaHCO 3 solution washing (20mL×2), the organic layer was washed with anhydrous Na 2 SO 4 Dry and concentrate under reduced pressure to obtain crude product. Recrystallization was further purified to obtain 1.320 g of pure benzimidazole compounds with a yield of 91%.
Embodiment 2
[0037] Add 1.080 g (10.0 mmol) of o-phenylenediamine, 1.272 g (12.0 mmol) of benzaldehyde, 0.032 g of potassium iodide and 1.00 mL of ethylene glycol into the conical flask in sequence. Microwave was used to heat indirectly 10 times, each heating was 50 seconds, and after heating, the Erlenmeyer flask was placed in the air, and the heating was continued after the temperature of the reaction mixture dropped. After the reaction was complete, the resulting mixture was dissolved in ethyl acetate, and then saturated NaHCO 3 solution washing (20mL×2), the organic layer was washed with anhydrous Na 2 SO 4 Dry and concentrate under reduced pressure to obtain crude product. Recrystallization was further purified to obtain 1.552 g of pure benzimidazole compounds with a yield of 80%.
Embodiment 3
[0039] Add 0.810 g (6.6 mmol) of 4-methyl-o-phenylenediamine, 0.954 g (9.0 mmol) of benzaldehyde, 0.081 g of potassium iodide and 0.66 mL of DMF into the Erlenmeyer flask in sequence. Microwave was used to heat indirectly 12 times, each time for 40 seconds. After heating, the Erlenmeyer flask was placed in the air, and the heating was continued after the temperature of the reaction mixture dropped. After the reaction was complete, the resulting mixture was dissolved in ethyl acetate, and then saturated NaHCO 3 solution washing (20mL×2), the organic layer was washed with anhydrous Na 2 SO 4 Dry and concentrate under reduced pressure to obtain crude product. Recrystallization was further purified to obtain 1.181 g of pure benzimidazole compounds with a yield of 86%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com