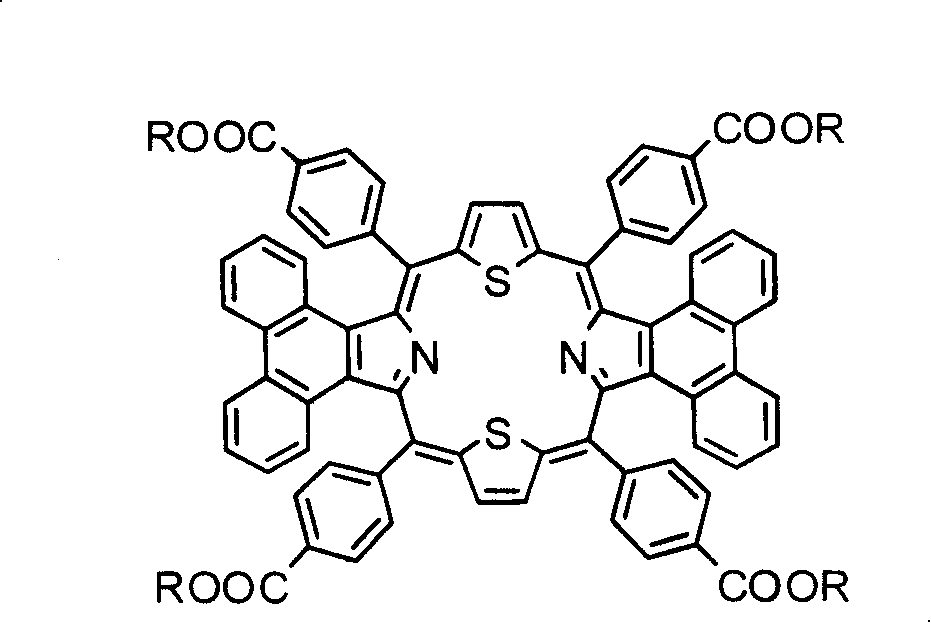
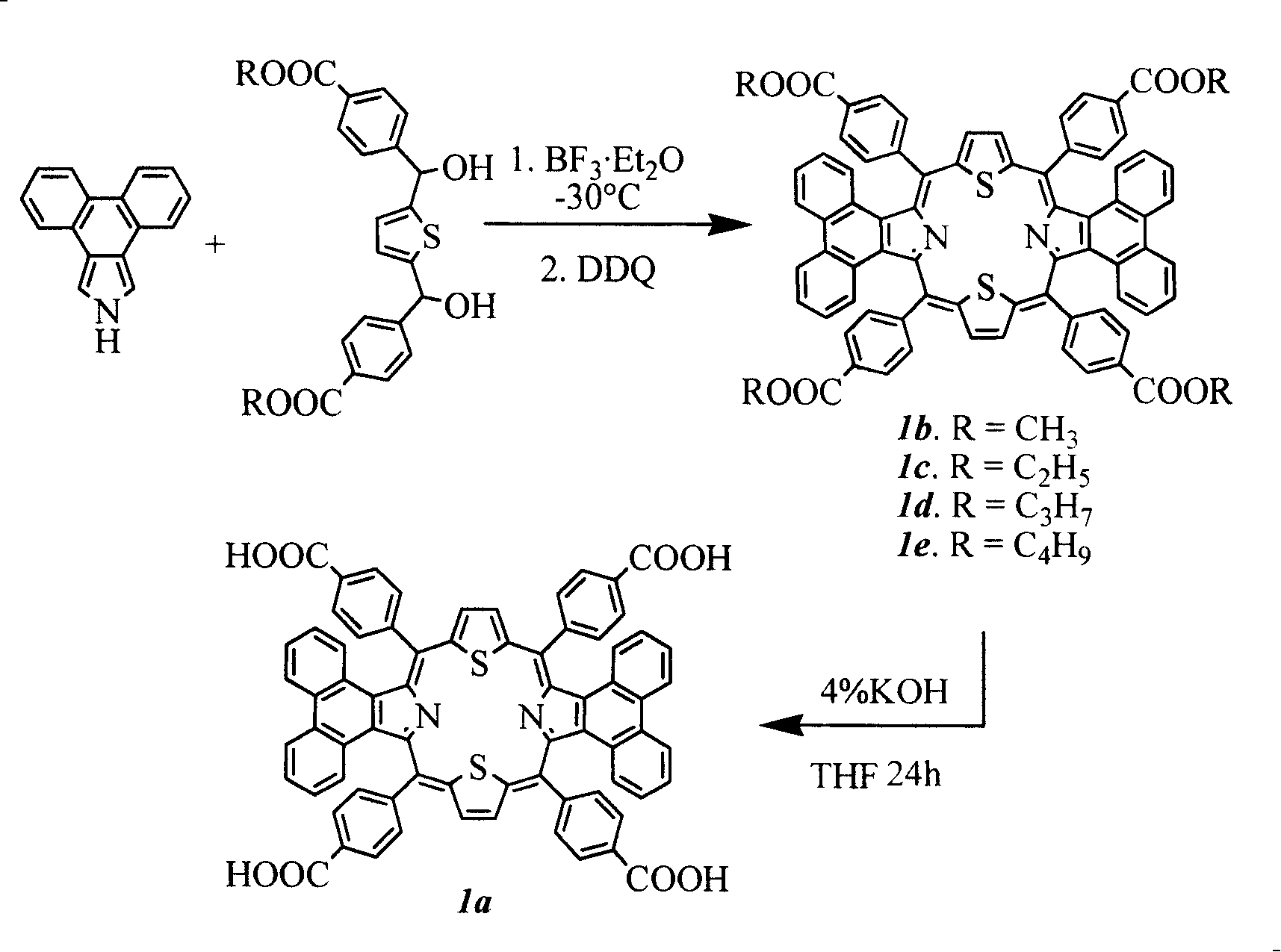
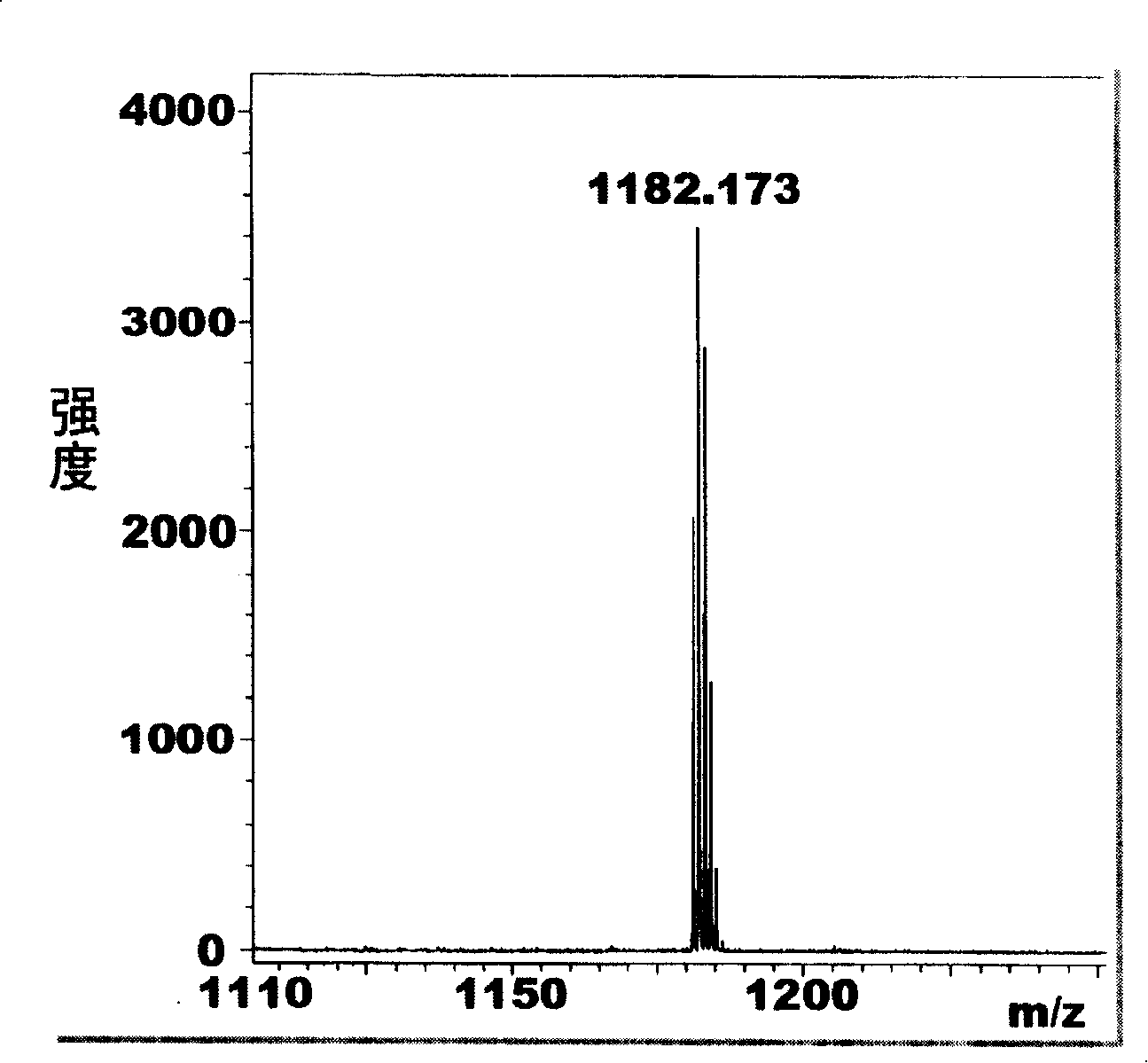
Method for synthesizing visible/near infrared porphyrin used for dyestuff sensitization solar energy battery and application thereof
A compound, dithioporphyrin technology, applied in the field of sensitizers, can solve problems such as failure to reach, and achieve the effect of improving absorption rate and conversion efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1: 5,10,15,20-tetrakis(4-methyl carboxylate)phenyl-diphenanthrene[9,10-b:9,10-l]-22,24-dithioporphyrin ( Synthesis of 1b):
[0043] Add 1mmol (412mg) 2,5-bis((4-benzoic acid ethyl ester) hydroxymethyl) thiophene, 1mmol (217mg) phenanthropyrrole and 100ml anhydrous dichloromethane in a 250ml round bottom flask, put the magnetic Start stirring, put the reaction bottle into a low-temperature device under the protection of argon and avoid light, control the reaction temperature at -30°C, and add a total of 80 μl of catalyst BF 3 ·Et 2 O, make it react at low temperature for 1 hour, then allow it to naturally heat up to 25° C., and continue the reaction for 48 hours. 1 mmol (227 mg) of DDQ was added to the reaction solution and reacted for 2 hours. The solvent was distilled off under reduced pressure for chromatographic separation, and purple-black crystal 1 was obtained after recrystallization from methanol and chloroform, with a yield of 15%; melting point > ...
Embodiment 2
[0044] Example 2: 5,10,15,20-tetrakis(4-methylbenzoate)phenyl-diphenanthrene[9,10-b:9,10-l]-22,24-dithioporphyrin Synthesis of (1b):
[0045] The method is the same as in Example 1, except that the reaction solvent dichloromethane is replaced by chloroform to carry out the reaction operation, and the yield is 15%.
Embodiment 3
[0046] Example 3: 5,10,15,20-tetrakis(4-methylbenzoate)phenyl-diphenanthrene[9,10-b:9,10-l]-22,24-dithioporphyrin Synthesis of (1b):
[0047] The method is the same as in Example 1, except that the amount of the reaction solvent dichloromethane is changed to 50ml for the reaction operation, and the productive rate is 13%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com