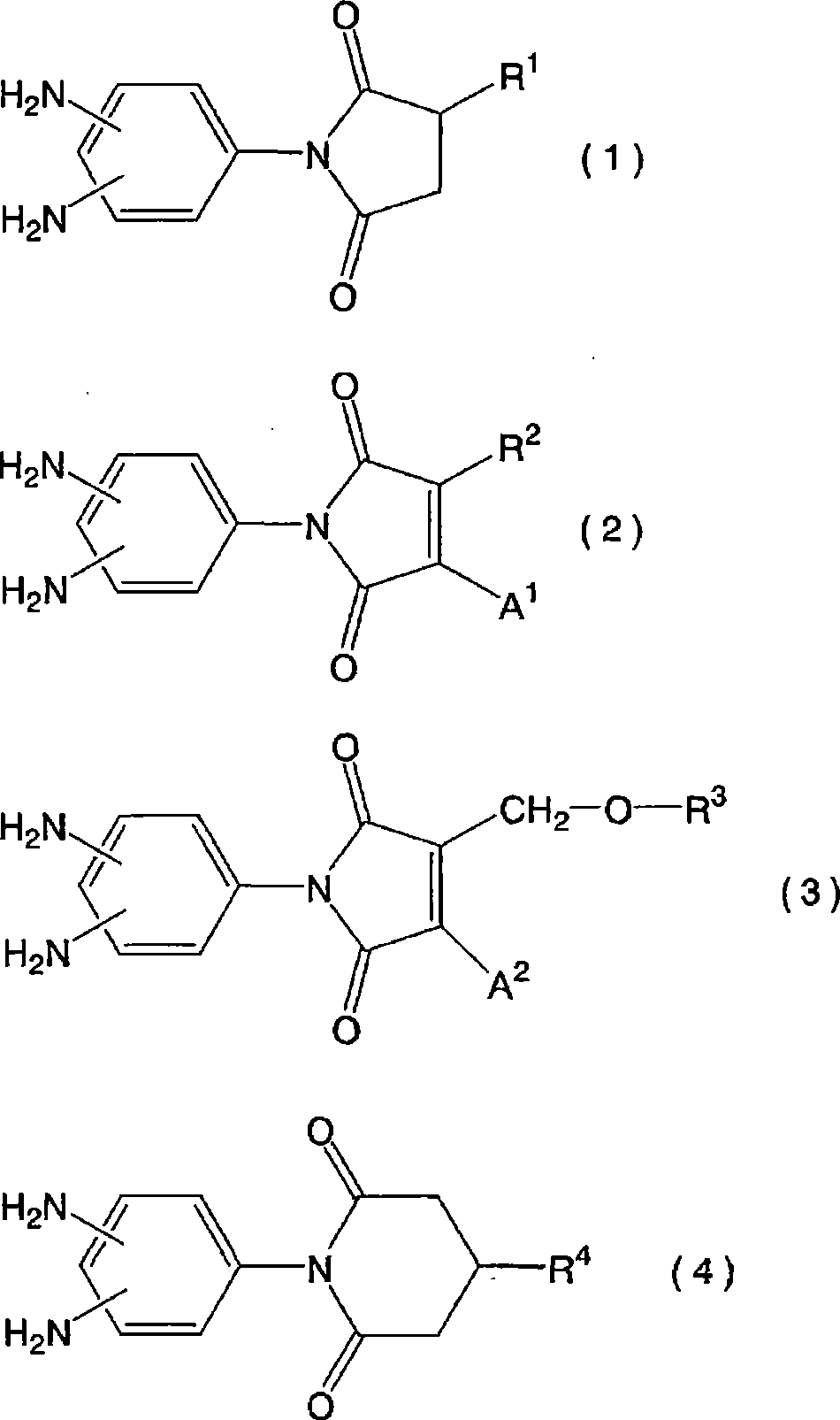
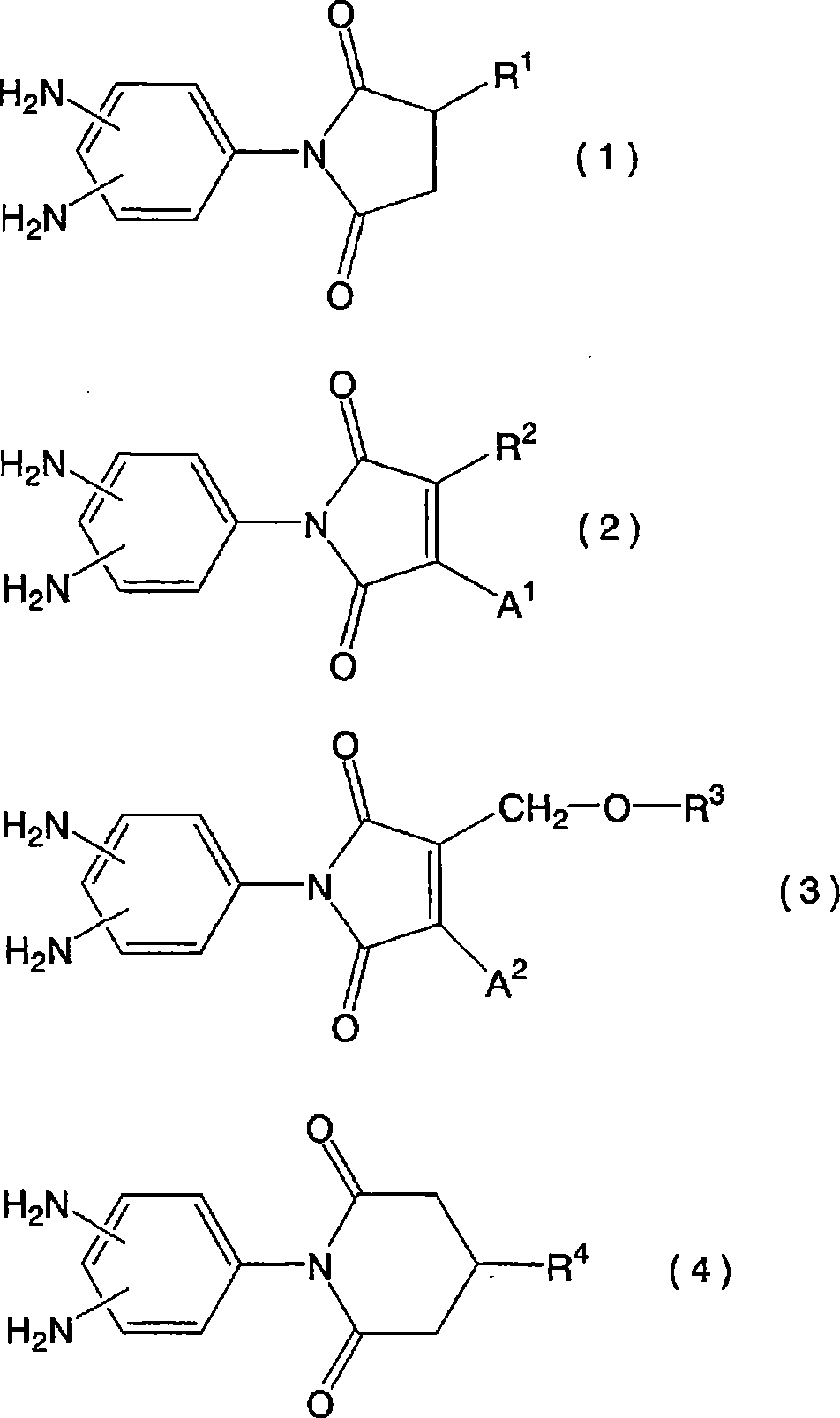
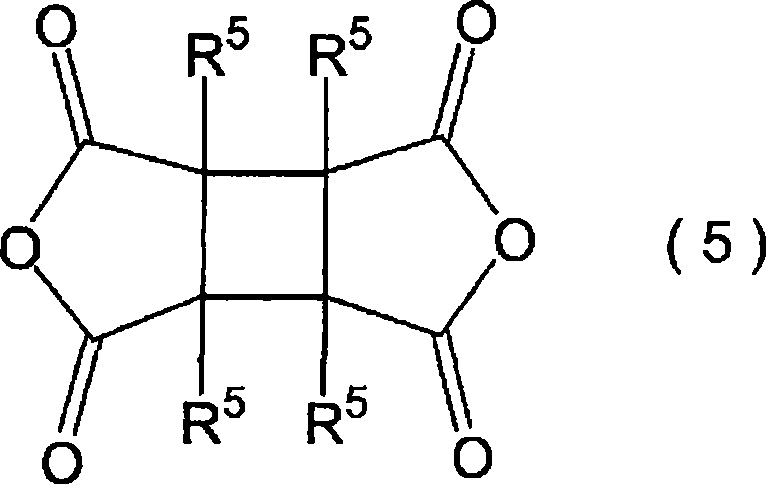
Liquid crystal aligning agnet and liquid crystal display element
A liquid crystal aligning agent and polymer technology, applied in liquid crystal materials, chemical instruments and methods, optics, etc., can solve the problems of unstable electrical properties, uneven pretilt angle, display spots or poor display, etc., and achieve low surface free energy. , high durability, excellent operation and processing adaptability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0132] Monomer Synthesis Example 1 (Synthesis of 1-(3,5-diaminophenyl)-3-octadecylsuccinimide)
[0133] Add 12.81 g (0.07 mol) of 3,5-dinitroaniline and 70 ml of acetic acid to a 300 ml three-necked flask ventilated with nitrogen, and stir while feeding nitrogen to dissolve the solid matter. 24.64 g (0.07 mol) of octadecyl succinic anhydride was added thereto, and it was made to react under reflux for 20 hours under nitrogen. After cooling the reaction solution to room temperature, 70 ml of methanol was added and allowed to stand overnight. The precipitated solid was filtered off, washed with methanol, and dried to obtain 30 g (83% yield) of 1-(3,5-dinitrophenyl)-3-octadecylsuccinimide.
[0134] Then, in a 500ml flask ventilated by nitrogen, add 30g (0.058mol) of the above-synthesized 1-(3,5-dinitrophenyl)-3-octadecylsuccinimide, 100ml ethanol, 100ml Tetrahydrofuran (THF) and 25 g of the reduction catalyst palladium carbon (Pd / C) were stirred at 70° C. for 1 hour. 42.5 ml (...
Synthetic example 2
[0135] Monomer Synthesis Example 2 (Synthesis of 1-(3,5-diaminophenyl)-3-dodecylsuccinimide)
[0136]Except using 18.76g (0.07mol) dodecyl succinic anhydride instead of 24.64g (0.07mol) octadecyl succinic anhydride, operate in the same way as in Monomer Synthesis Example 1 to obtain 11g (0.030mol, yield 51% ) 1-(3,5-diaminophenyl)-3-dodecylsuccinimide.
Synthetic example 3
[0137] Monomer Synthesis Example 3 (Synthesis of 1-(3,5-diaminophenyl)-3-heptadecyl-4-methylmaleimide)
[0138] Add 31.5g (0.25mol) dimethyl maleic anhydride, 89.0g (0.5mol) N-bromosuccinimide, 1.0g (4.15mmol) diphenyl peroxide to a 2000ml three-necked flask ventilated by nitrogen formyl and 1500ml carbon tetrachloride, heated to reflux for 5 hours. The reaction solution was cooled to room temperature, left to stand overnight at room temperature, and then filtered. After the filtrate was washed with water, the organic layer was concentrated in a rotary evaporator. The resulting oily crude product was distilled under high vacuum (120-125° C. / 2 mmHg) to obtain 20.0 g (0.1 mol, yield 39%) of intermediate 3-bromomethyl-4-methylmaleic anhydride.
[0139] Then, add 16.4g (80mmol) above-mentioned 3-bromomethyl-4-methyl maleic anhydride, 1.52g (8.0mmol) copper iodide, 400ml ether and 160ml of HMPA (hexamethylphosphoric triamide), cooled to -5 ~ 0 ° C under the condition of feeding ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface tension | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com