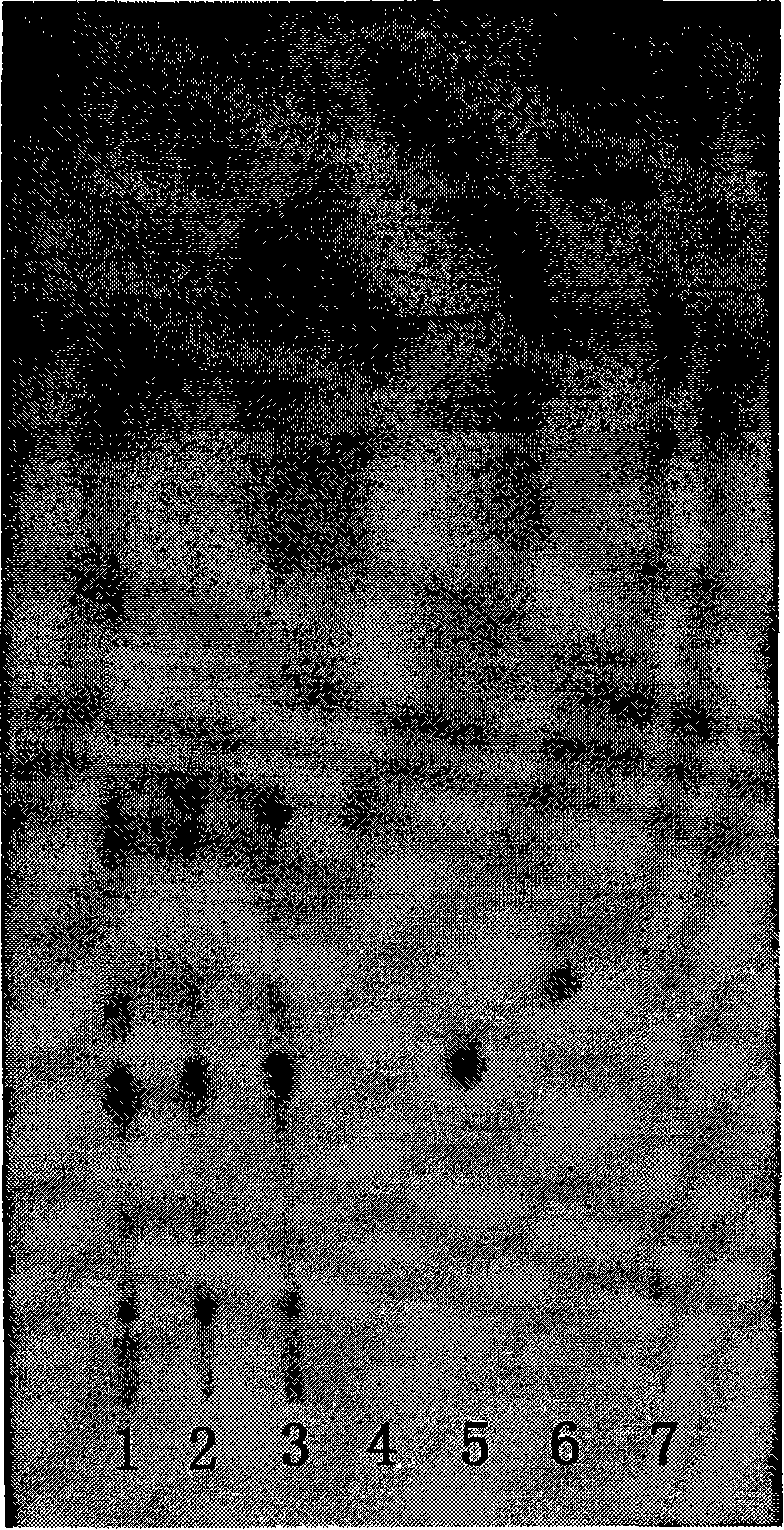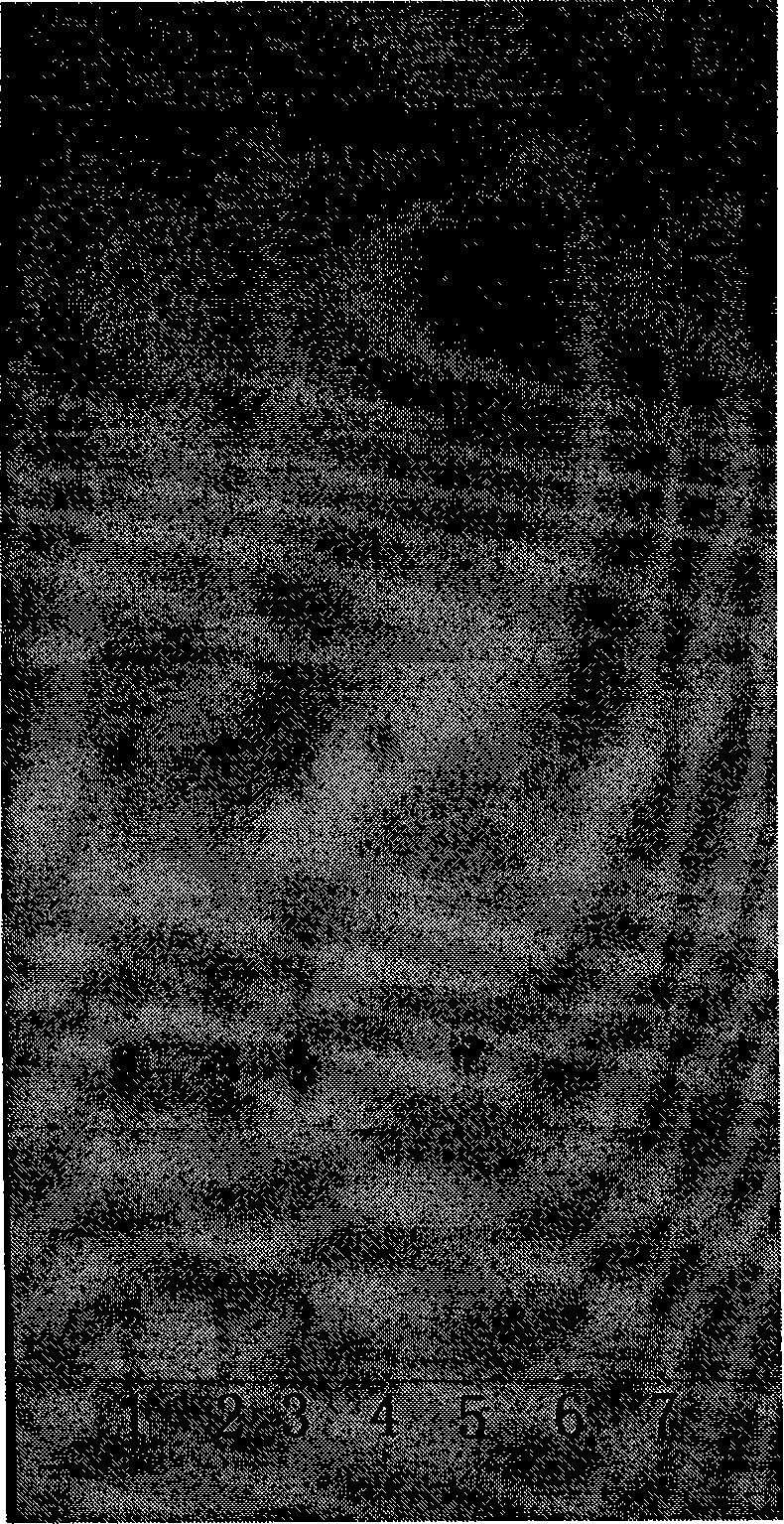Preparations quality control method containing radix dipsaci and pseudo-ginseng activity extracting combination
A quality control method and composition technology, applied in the field of quality control of natural medicines, can solve problems such as the lack of quality research of Sanqi compound preparations, achieve good reproducibility and reliability, good recovery rate, and quality control effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] 1.1 Expansion conditions
[0049] Stationary phase: silica gel G plate (100×200mm, thickness 0.20-0.25mm, Qingdao Ocean Chemical Factory Branch).
[0050] Developing solvent: use the lower layer solution of chloroform-methanol-n-butanol-water (2:1:3:3) as the developing solvent, develop at not lower than 20°C for a saturation time of 30 minutes.
[0051] Color development: Spray with sulfuric acid ethanol solution (1→10), and immediately heat at 100-110°C until the spots are clear in color. 1.2 Solution preparation
[0052] Preparation of the test solution Take 380 mg of the compound capsule content, put it in a 10 ml measuring bottle, add methanol to the volume, ultrasonicate for 5 minutes, shake well, filter, and obtain.
[0053] Preparation of aketoside D reference solution Take 30 mg of aketoside D, put it in a 10ml measuring bottle, add methanol to dissolve, dilute, constant volume, shake well, and get ready.
[0054] Ginsenoside Rb 1 Ginsenoside Rb was used fo...
Embodiment 2
[0063] 1. Instruments and Materials
[0064] Agilent 1100 series high performance liquid chromatography, including binary gradient pump, column thermostat, ultraviolet detector; HW chromatographic workstation (Shanghai Qianpu); ZRS-8G drug dissolution apparatus (Tianjin University Radio Factory); KQ2200DE medical numerical control Ultrasonic cleaner; needle filter (water film): 0.45μm, Germany MENBRANA company
[0065] Methanol (analytically pure), acetonitrile (chromatographically pure, German Merk Company), distilled water (self-made).
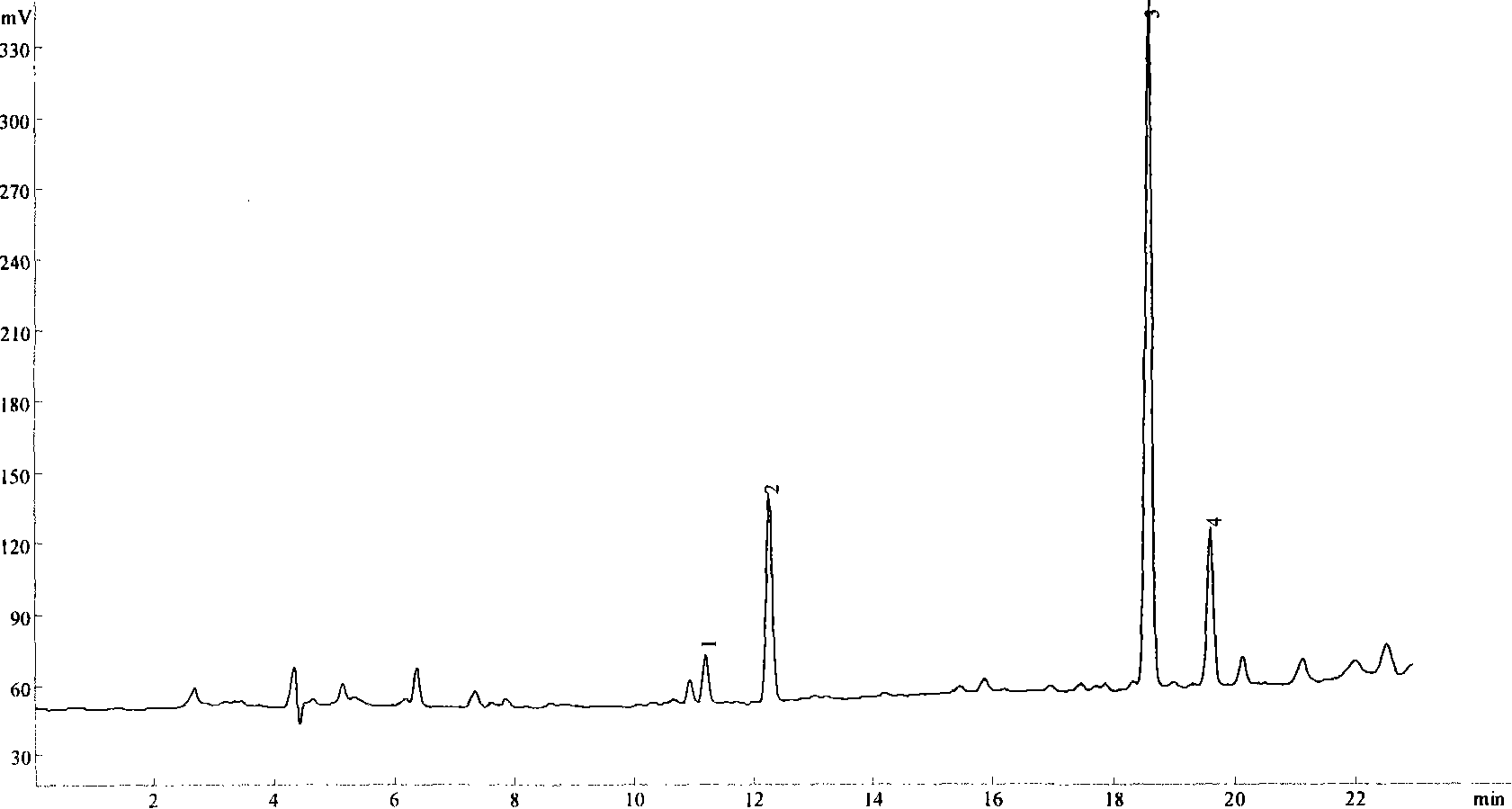
[0066] 2. Chromatographic conditions
[0067] Column: Kromasil C 18 Column (250mm×4.6mm, 5um); acetonitrile-water is the mobile phase, wherein A is acetonitrile, B is water, A+B=100%, adopts gradient elution; 0~20 minutes, 80%~60% B, 20-21 minutes, 60%-80% B, maintain for 5 minutes, record the chromatogram. The flow rate is 0.8ml / min; the detection wavelength is 203nm.
[0068] 3. Experimental method
[0069] 3.1 Solution Preparation ...
Embodiment 3
[0087] 1. Instruments and Reagents
[0088] Instruments: Shimadzu UV-2401 Spectrophotometer; Shimadzu MPS-2000 Spectrophotometer; BRANSON200 Ultrasonic Cleaner (ULTRASONIC CLEANER, 50-60HZ); WH-1 Micro Vortex Mixer (Shanghai Huxi Analytical Instrument Factory)
[0089] Reagent: Methanol (analytical grade, Nanjing Chemical Reagent Factory); 77% sulfuric acid (concentrated sulfuric acid is superior grade, 23ml distilled water adds sulfuric acid 77ml); 8% vanillin (vanillin) ethanol solution (analytical grade, 800mg vanillin is dissolved in 10ml in absolute ethanol, prepared just before use).
[0090] 2. Test method
[0091] 2.1 Solution preparation
[0092] Preparation of the test solution: Accurately weigh 15 mg of the test powder of the compound capsule, place it in a 100ml measuring bottle, add methanol to volume, ultrasonically aid in dissolution for 5 minutes, let it cool to room temperature, shake well, filter, and obtain.
[0093] Preparation of reference substance sol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com