Fusidate sodium composition and preparation of its freeze-drying preparations
A technology of sodium fusidate and its composition, which is applied in the field of pharmaceutical preparations, can solve problems such as poor long-term stability, increased foreign matter, and large material changes, and achieve the effects of improved tolerance, good stability, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
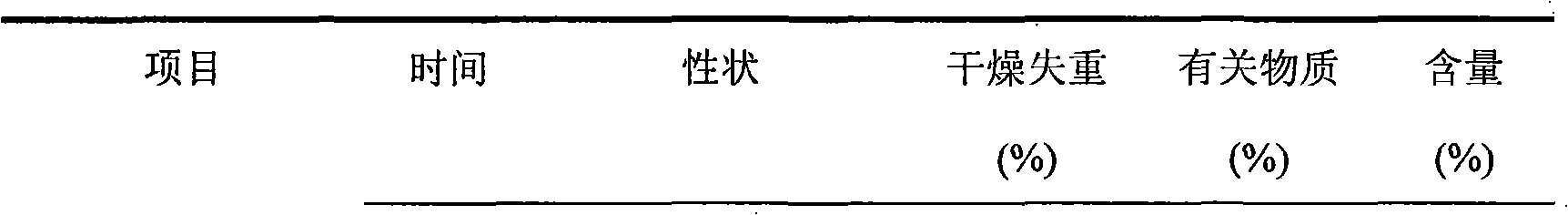
Embodiment 1
[0031] Each 1000 vials of lyophilized preparation contains the following ingredients:
[0032] Sodium Fusidate 125g
[0033] Mannitol 20g
[0034] Arginine 5g
[0035] Weigh the prescribed amount of mannitol and arginine, add 2000ml of water for injection, stir to dissolve, the temperature of the solution drops below 60°C, then add the prescribed amount of sodium fusidate, stir continuously to dissolve completely, add the prepared total Amount of 0.5% (W / V) activated carbon for injection, stirred and left for 25 minutes, decarburized by filtration, added water for injection to the full amount and set aside. Fine filter the above solution with a 0.22 μm microporous membrane until the filtrate is clear. After the semi-finished product passes the inspection, it is subpackaged and filled into 1,000 10ml vials so that the main drug content is 125mg, and frozen at low temperature (-40°C). 3 hours, primary drying temperature -25°C for 4 hours, 0°C for 7 hours, 18°C for 7 hours, ...
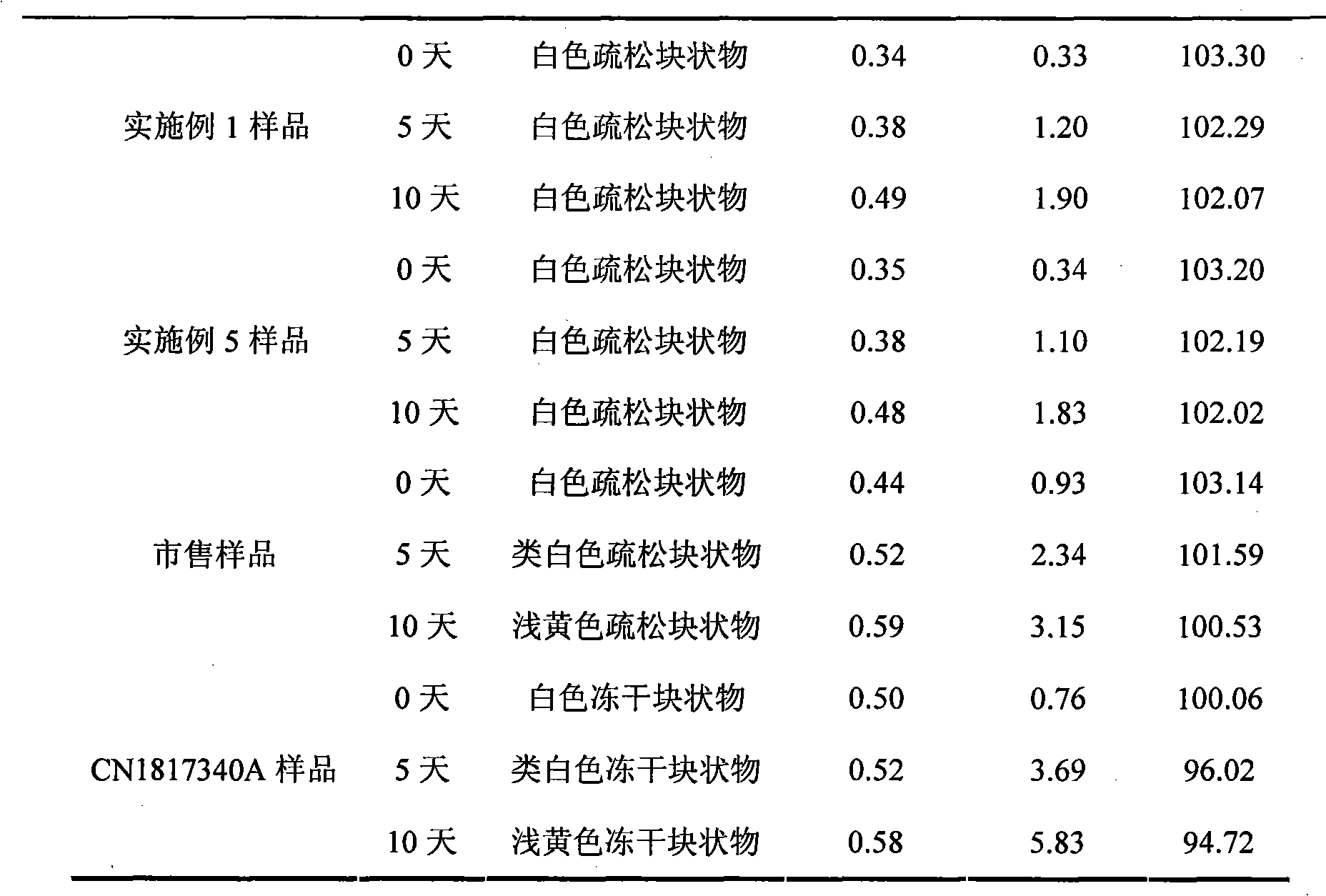
Embodiment 2
[0037] Each 1000 vials of lyophilized preparation contains the following ingredients:
[0038] Sodium Fusidate 125g
[0039] Mannitol 20g
[0040] Arginine 100g
[0041] Disodium edetate 1g
[0042] The raw materials and auxiliary materials of the above prescription were prepared into 1000 bottles of sodium fusidate freeze-dried preparation according to the method of Example 1.
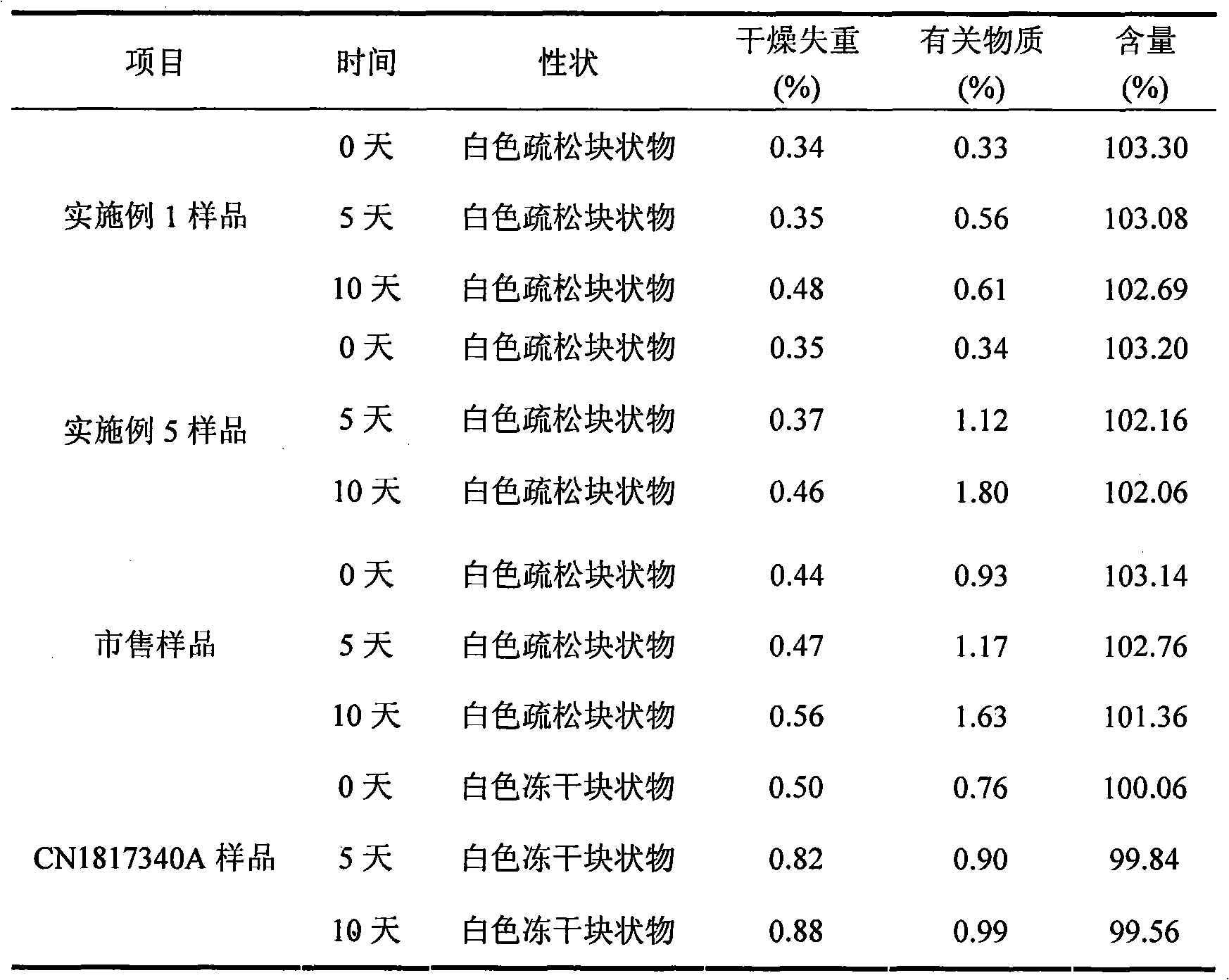
Embodiment 3
[0044] Each 1000 vials of lyophilized preparation contains the following ingredients:
[0045] Sodium Fusidate 125g
[0046] Mannitol 150g
[0047] Arginine 5g
[0048] Disodium edetate 1g
[0049] The raw materials and auxiliary materials of the above prescription were prepared into 1000 bottles of sodium fusidate freeze-dried preparation according to the method of Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com