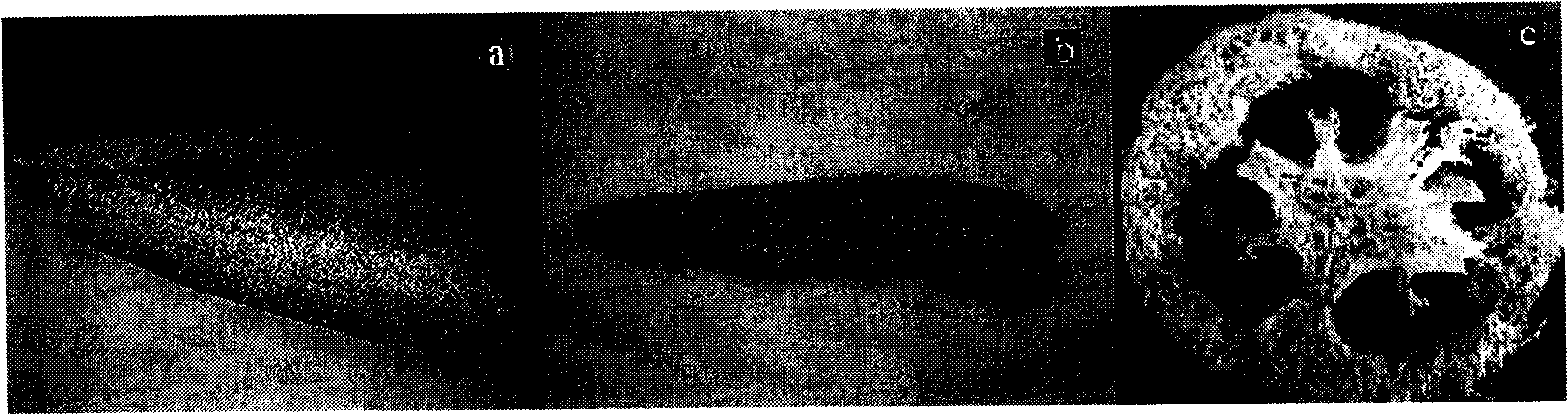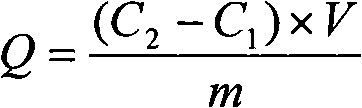Application of luffa as adsorbent in metallic ion adsorption
A technology of metal ions and loofah, applied in the direction of adsorption water/sewage treatment, other chemical processes, chemical instruments and methods, etc., can solve the problems of high development cost, secondary pollution, environmental pollution, etc., achieve low price and increase income , Environmentally friendly effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: the method for static adsorption carries out water treatment
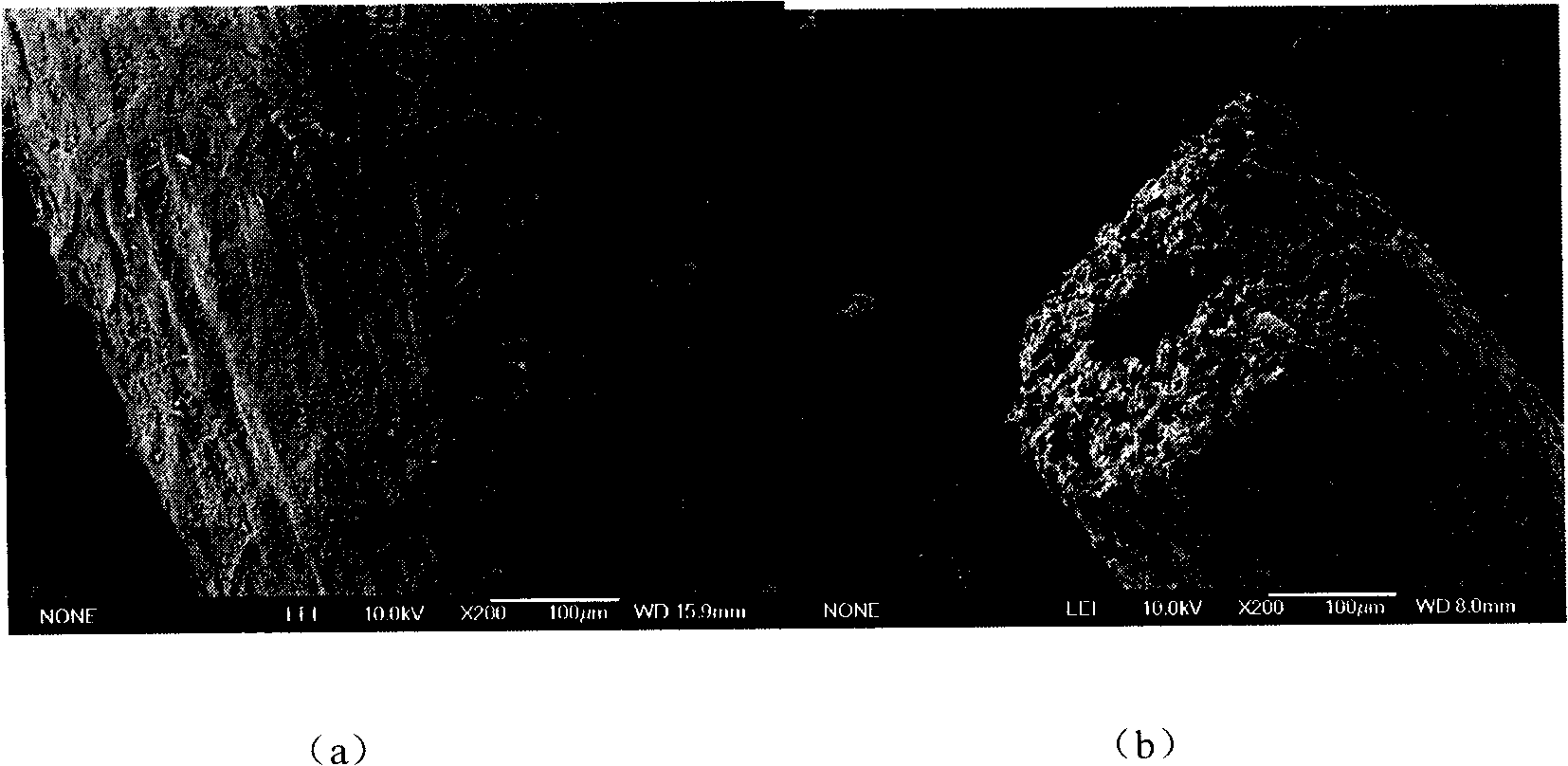
[0026] Cut the natural loofah into small pieces, and measure its adsorption capacity for mixed metal ions. The method is: (1) Take 1.5g of loofah, transfer it to a three-necked bottle, add 150mL of metal ion mixed solution, and drop an appropriate amount of HCl or HAc , adjust the pH value of the mixed solution to 3-4. Stir at a temperature of 70°C for 6 hours. The loofah was washed three times with deionized water and dried at 80°C. for atomic emission spectrometry.
[0027] (2) In the adsorption experiment, three different mixed solutions of metal ions were used:
[0028] a) contains Ca 2+ 、Na + 、Cu 2+ , Mn 2+ 、Ba 2+ , Fe 3+ , Zn 2+、Cd 2+ Hydrochloride solution (the concentration of each metal ion is about 0.01M)
[0029] b) Contains ZnCl 2 , Pb(Ac) 2 , CdCl 2 The mixed solution (the concentration of each metal ion is about 0.005M)
[0030] c) Contains Pb(Ac) 2 , Co(NO 3 ) ...
Embodiment 2
[0044] Embodiment 2: Atomic Emission Spectroscopy (AES) investigates its adsorption-desorption reaction
[0045] (1) Weigh 4 portions of about 1.00g loofah, place them in 1#, 2#, 3#, 4# three-neck bottles, add 100mL Pb(Ac) respectively 2 , CuCl 2 , ZnCl 2 , FeCl 3 Adsorption is carried out in four kinds of single metal ion solutions, the solution concentration is 1.4mmol, 1.2mmol, 5.3mmol, 2.9mmol in turn, and an appropriate amount of HCl or HAc is added dropwise to adjust the pH value to 3~4, and the adsorption is in the range of 40~45℃ 20 hours.
[0046] (2) Discard the reaction solution in each three-neck bottle, rinse the loofah with an equal amount of deionized water (about 100 mL), and then put the loofah into an oven to dry at 80°C.
[0047] (3) Add 200mL deionized water into the three-necked bottle, desorb in a water bath at 80-85°C for 3 hours, then replace with 200ml deionized water, and continue the water bath for 3 hours.
[0048] (4) Take out the desorbed loo...
Embodiment 3
[0050] Embodiment 3: Atomic Absorption Spectroscopy (AAS) investigates its effect on Zn 2+ and Cu 2+ The adsorption-desorption reaction of:
[0051] (1) Weigh 2 portions of 0.4g loofah respectively, put them in two 100ml beakers, numbered 1#, 2#. Add 5mmol Zn-containing 2+ and Cu 2+ 40mL of a single metal ion solution, adjust the pH value to 4-6, and let it stand for adsorption at room temperature for 24 hours.
[0052] (2) Collect the filtrate in the 1# beaker into a 100mL volumetric flask, wash the loofah twice with deionized water, 10mL each time, and put the filtrate into the volumetric flask. Use atomic absorption spectrometry to measure its residual ion concentration, see Figure 3, and then convert it into the adsorption amount of loofah on metal ions.
[0053] (3) Discard the solution in the 2# beaker, and wash the loofah twice with deionized water, 10 mL each time. Transfer the 2# loofah into 250mL three-neck bottles respectively, add 80mL of HCl with a pH value ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com