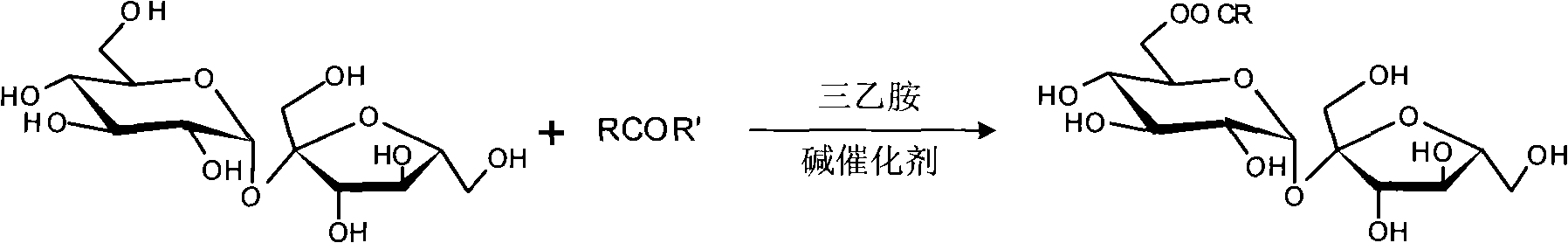
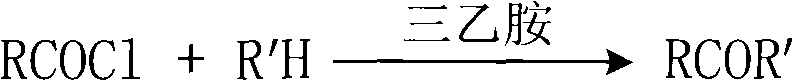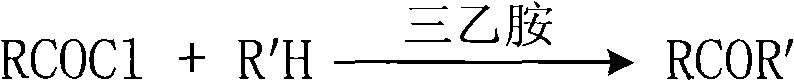Process for selectively synthesizing sucrose-6-ester
A selective, sucrose-based technology, applied in chemical instruments and methods, sugar derivatives, sugar derivatives, etc., can solve the problems of difficult organotin recovery, cumbersome operation, complicated process, etc., and achieves convenient and easy synthesis method and excellent preparation process. Simplicity and the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: Synthesis of sucrose-6-acetate with N-acetyl-2-thiazolethione
[0025] 1.1 Synthesis of N-acetyl-2-thiazolethione
[0026] Add 10mmol 2-thiazolethione and 25mL dichloromethane into a 150mL three-neck flask, stir to dissolve, add 2.4mL triethylamine, stir and cool to 0°C under an ice-water bath. Then, a mixture of 15 mmol acetyl chloride and 5 mL of anhydrous dichloromethane was added dropwise to the reaction solution, and the reaction temperature was kept below 5° C. during the dropwise addition. After the dropwise addition, it was raised to room temperature and reacted for 2 hours. TLC monitoring showed that the reaction was completed. Add 0.5N hydrochloric acid solution to neutralize, then add 5% sodium bicarbonate solution to wash until neutral, separate the liquids to obtain an organic phase, and dry with anhydrous magnesium sulfate. , filtered, and the solvent was distilled off to obtain N-acetyl-2-thiazolethione as a light yellow liquid with a crude yi...
Embodiment 2
[0029] Example 2: Synthesis of sucrose-6-propionate with N-propionyl-2-thiazolethione
[0030] 2.1 Synthesis of N-propionyl-2-thiazolethione
[0031] Add 10mmol 2-thiazolethione and 25mL dichloromethane into a 150mL three-neck flask, stir to dissolve, add 2.4mL triethylamine, stir and cool to 0°C under an ice-water bath. Then, a mixture of 15 mmol propionyl chloride and 5 mL of anhydrous dichloromethane was added dropwise to the reaction solution, and the reaction temperature was kept below 5° C. during the dropwise addition. After the dropwise addition, the mixture was raised to room temperature and reacted for 2 hours. TLC monitoring showed that the reaction was complete. Add 0.5N hydrochloric acid solution to neutralize, then add 5% sodium bicarbonate solution to wash until neutral, separate the liquids to obtain an organic phase, and dry it with anhydrous magnesium sulfate. After filtration, the solvent was distilled off to obtain N-propionyl-2-thiazolethione as light yel...
Embodiment 3
[0034] Example 3: Synthesis of sucrose-6-acetate with N-acetylbenzotriazole
[0035] 3.1 Synthesis of N-acetylbenzotriazole
[0036] Add 10mmol benzotriazole and 25mL dichloromethane into a 150mL three-neck flask, stir to dissolve, add 2.4mL triethylamine, stir and cool to 0°C under an ice-water bath. Then, a mixture of 15 mmol acetyl chloride and 5 mL of anhydrous dichloromethane was added dropwise to the reaction solution, and the reaction temperature was kept below 5° C. during the dropwise addition. After the dropwise addition, the mixture was raised to room temperature and reacted for 2 hours. TLC monitoring showed that the reaction was complete. Add 0.5N hydrochloric acid solution to neutralize, then add 5% sodium bicarbonate solution to wash until neutral, separate the liquids to obtain an organic phase, and dry it with anhydrous magnesium sulfate. After filtration, the solvent was distilled off to obtain N-acetylbenzotriazole in the form of light yellow liquid, with a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com