Petroleum naphtha reforming catalyst and preparation method thereof
A technology for reforming catalysts and catalysts, which is applied in the reforming of naphtha and the petroleum industry. It can solve the problems of catalyst carrier pore size enlargement, surface area reduction, and mechanical strength deterioration, so as to improve catalytic performance, reduce carbon deposits, Effect of Liquid Yield and Aromatics Yield Increase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] The preparation method of the catalyst provided by the invention comprises the steps of impregnating the carrier with an impregnating solution containing noble metal compounds and halogens, drying, and then performing activation treatment with air containing water and halogen compounds.
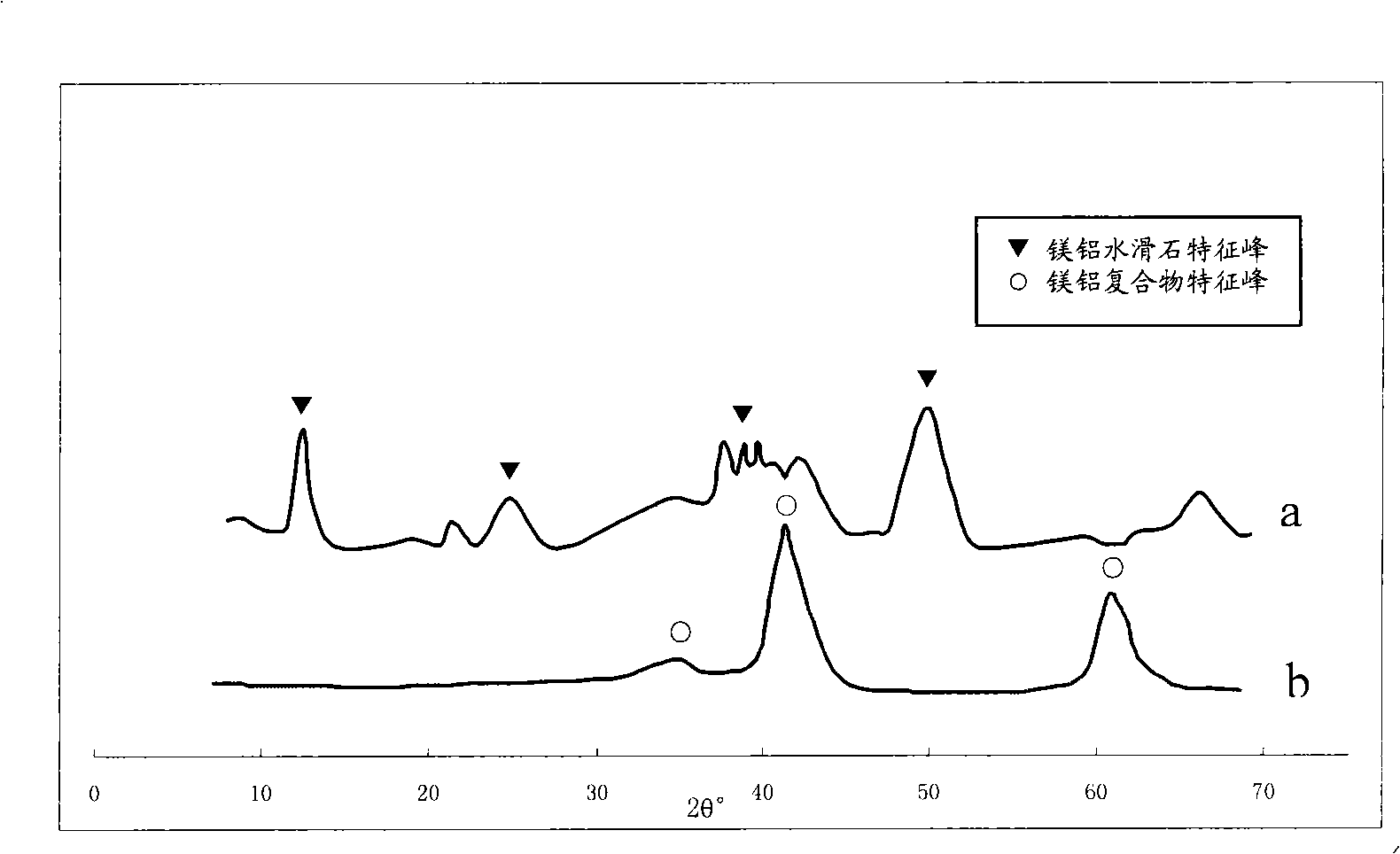
[0024] The preferred method for preparing the catalyst composite carrier in the present invention is: immerse the formed alumina carrier in the mixed solution prepared by the magnesium compound and the basic compound, fully react under the conditions of 0.1-0.5MPa and 100-150°C, collect the solid and wash , After drying, calcining at 500-650°C to obtain a composite carrier. The above method is a method of forming a Mg(Al)O covering layer on the surface of shaped alumina. The aluminum source for the synthesis of magnesium aluminum hydrotalcite is provided by the alumina itself, and the shaped alumina is put into a magnesium compound and a basic compound to make it In the mixed solution,...
example 1
[0039] Preparation of vectors described in the present invention.
[0040] (1) Preparation of spherical alumina
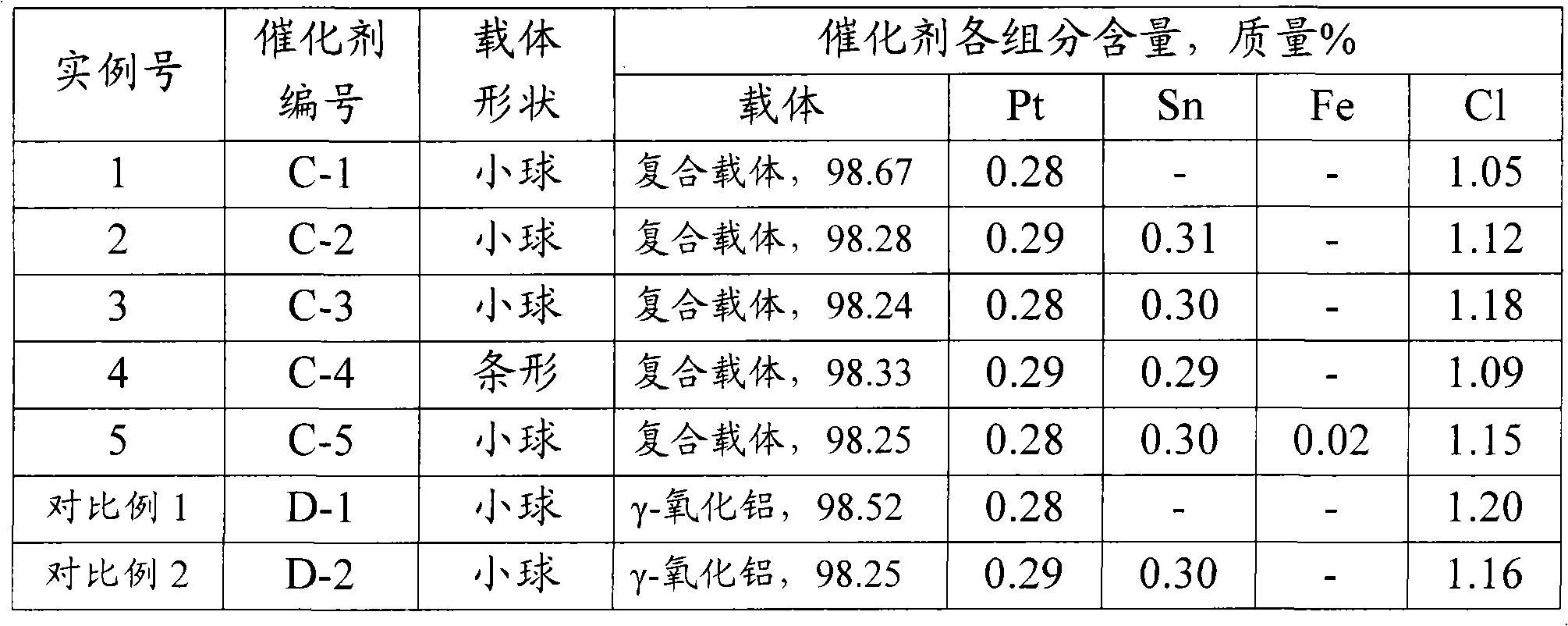
[0041] Take 100 grams of pseudo-boehmite powder (with an alumina content of 74.6% by mass, produced by Shandong Aluminum Co., Ltd.) in a beaker, add 100 grams of deionized water, and stir for 0.5 hours to make the two initially mixed and slurried. Add 5 ml of 1:1 nitric acid solution, stir and acidify for 0.5 hours. The above-mentioned acidified slurry was dropped into an oily ammonia column with 10 cm kerosene at the upper end and 8 mass % ammonia water at the lower end to form balls. The pellets sink to the bottom of the column, take them out after aging for 2 hours, wash with deionized water until the washing liquid is close to neutral, then dry the washed pellets at 110°C for 10 hours, and bake them at 550°C for 6 hours to obtain γ-alumina pellets. Ball A-1.
[0042] (2) Preparation of carrier containing magnesium-aluminum composite
[0043] Take 0.95 g of ...
example 2
[0048] (1) Preparation of tin-containing composite carrier.
[0049] Prepare tin-containing alumina by the method of example 1 (1) step, difference is that adding 37.6 milliliters of concentrations is the SnCl of 13.5 mg / ml in the acidification process 4 The solution was dried and calcined at 550° C. for 6 hours to obtain γ-alumina pellets A-2 with a tin content of 0.31% by mass.
[0050] Get the tin-containing gamma-alumina pellet A-2, prepare the surface layer as the tin-containing composite carrier B-2 of the magnesium-aluminum composite by the method of example 1 (2), wherein MgO and Al 2 o 3 The mass ratio is 1:200.
[0051] (2) Preparation of catalyst
[0052] Prepare catalyst by the method for example 1 (3) step, difference is that the carrier that uses is the composite carrier B-2 that contains tin, and the component content of the catalyst C-2 that makes is shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com