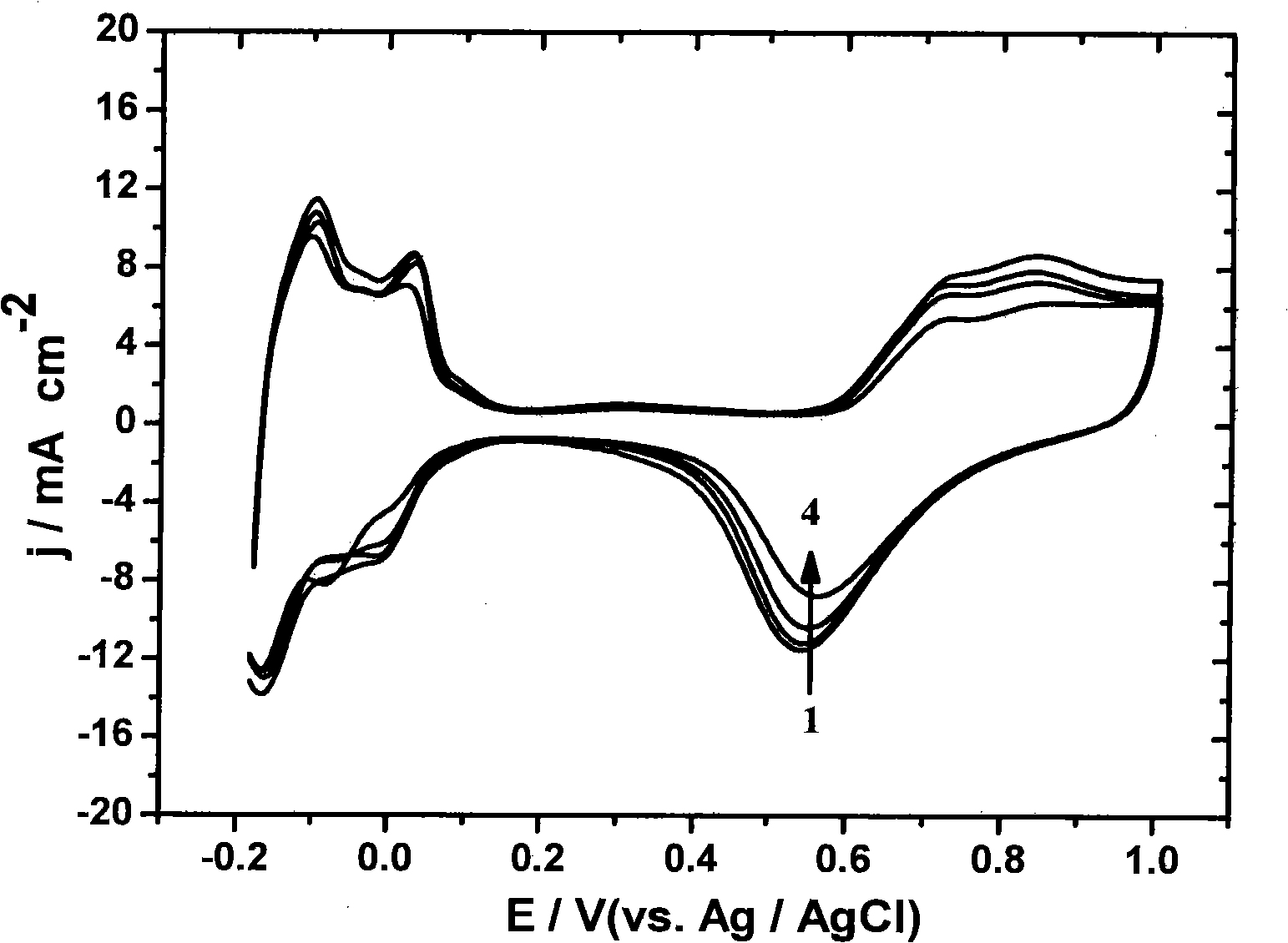
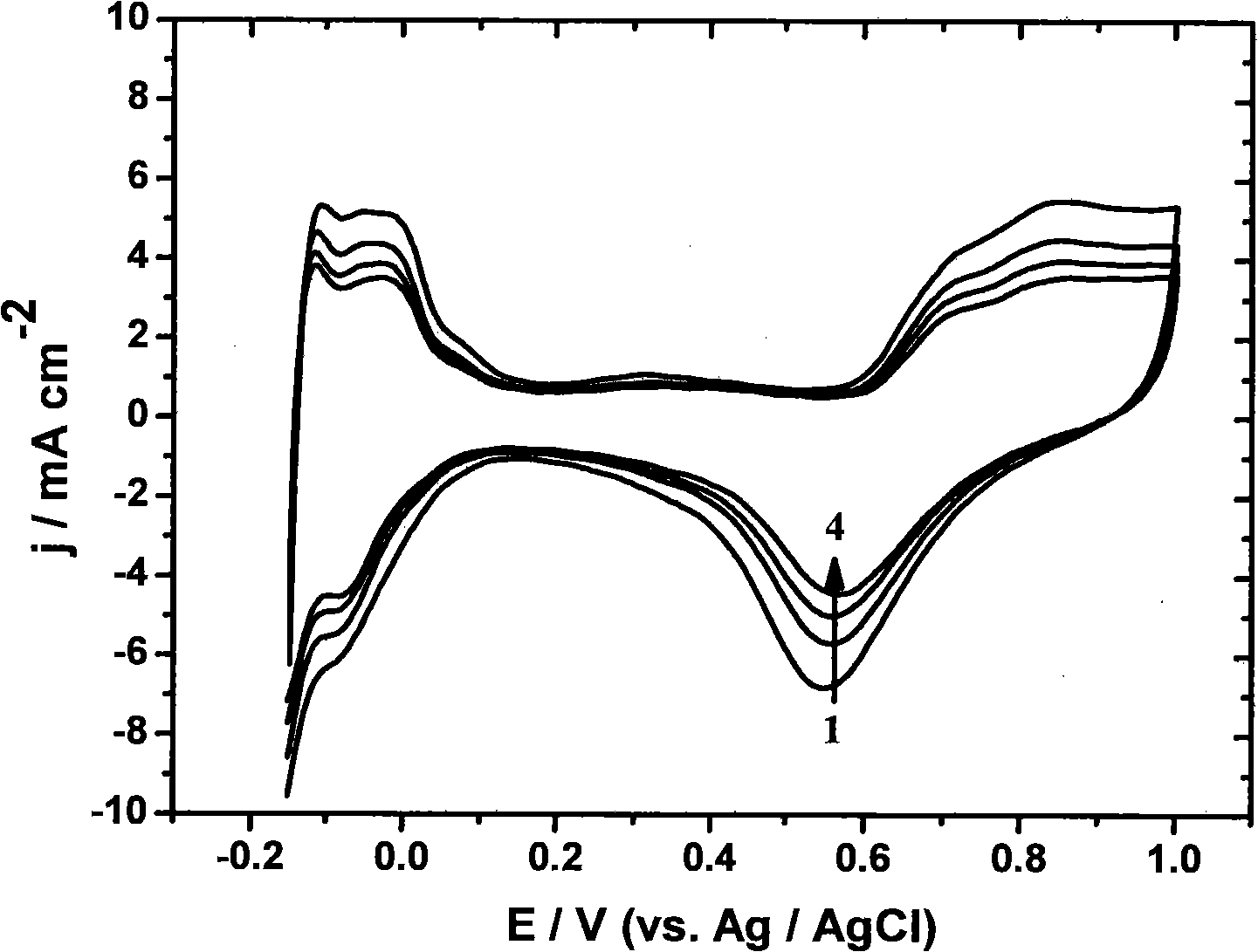
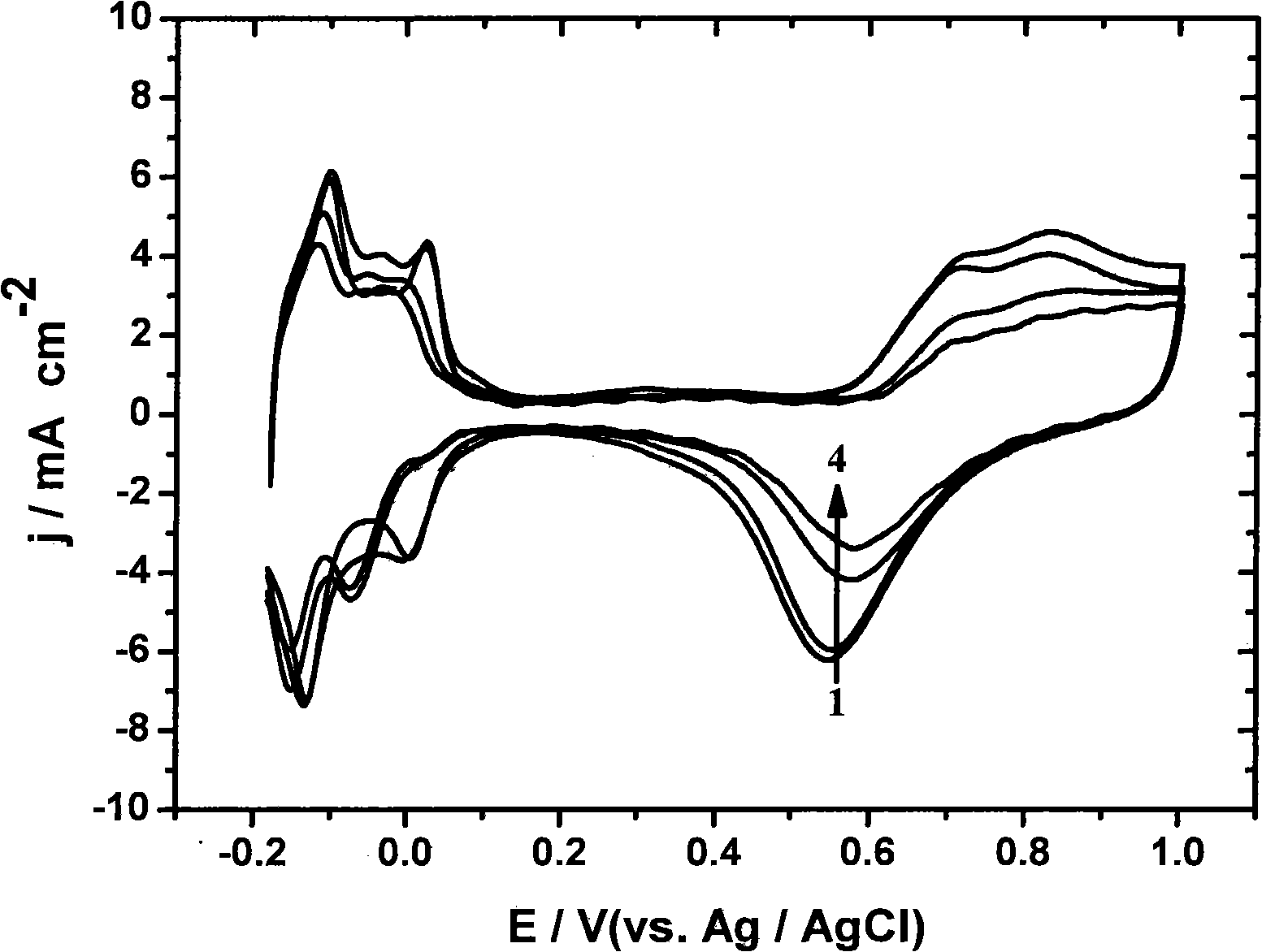
Method of transitional metal anchored platinum catalyst on carbon nano-tube
A carbon nanotube and platinum catalyst technology, applied in the field of fuel cells, can solve the problems of platinum-based catalyst stability and poor oxygen reduction activity, and achieve the effects of good catalyst stability, corrosion avoidance, and high oxygen reduction activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (1), purification of carbon nanotubes
[0035] Weigh 1g of commercially available carbon nanotubes (Shenzhen Bill Technology Co., Ltd.), add 160ml of concentrated nitric acid, heat and reflux for 3 hours, cool, dilute with ultrapure water, filter out the supernatant, wash centrifugally several times, dry, and grind to obtain Purified carbon nanotubes.
[0036] (2) Functionalization of carbon nanotubes
[0037] Weigh 1 g of purified carbon nanotubes, add 100 ml of 30% hydrogen peroxide and concentrated sulfuric acid mixed acid with a volume ratio of 1:4, stir with ultrasonic vibration for 3 hours, dilute with ultrapure water, and filter out the upper layer after standing for 24 hours The supernatant is washed by centrifugation several times, dried and ground to obtain functionalized carbon nanotubes.
[0038] (3) Preparation of nickel-anchored platinum catalyst on carbon nanotubes
[0039] According to functionalized carbon nanotubes: chloroplatinic acid: the mass rat...
Embodiment 2
[0045] Steps (1)-(2) are the same as steps (1)-(2) in Example 1.
[0046] (3) Preparation of nickel-anchored platinum catalyst on carbon nanotubes
[0047] According to the mass ratio of functionalized carbon nanotubes: potassium chloroplatinate: nickel chloride is 1: 2: 0.1, take functionalized carbon nanotubes, potassium chloroplatinate and nickel chloride, add to excess 1,5 - Add 30% potassium chloroplatinate, all functionalized carbon nanotubes and all nickel chloride to pentanediol solvent. Ultrasonic oscillation for 0.5 hours, then under the protection of nitrogen or argon atmosphere, stir and reflux in an oil bath at 250°C for 1 hour, then add the remaining 70% potassium chloroplatinate to the reflux device, and continue to stir and reflux under the above conditions Cool to room temperature after 3.5 hours. Then the product was centrifuged twice with 95% ethanol and ultrapure water, dried, and finally treated at 180° C. for 2 hours in a hydrogen atmosphere to obtain a...
Embodiment 3
[0049] Steps (1)-(2) are the same as steps (1)-(2) in Example 1.
[0050] (3) Preparation of iron-anchored platinum catalysts on carbon nanotubes
[0051] According to the mass ratio of functionalized carbon nanotubes: sodium chloroplatinate: ferric nitrate is 1: 1: 0.7, take functionalized carbon nanotubes, sodium chloroplatinate and ferric nitrate, add them to excess ethylene glycol solvent respectively Add 20% sodium chloroplatinate, all functionalized carbon nanotubes and all iron nitrate. Ultrasonic oscillation for 0.8 hours, then under the protection of nitrogen or argon atmosphere, stir and reflux in an oil bath at 180°C for 1.5 hours, then add the remaining 80% sodium chloroplatinate to the reflux device, and continue to stir and reflux under the above conditions Cool to room temperature after 2.5 hours. Then the product was centrifuged twice with 95% ethanol and ultrapure water, dried, and finally treated at 150° C. for 1.5 hours in a hydrogen atmosphere to obtain a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com