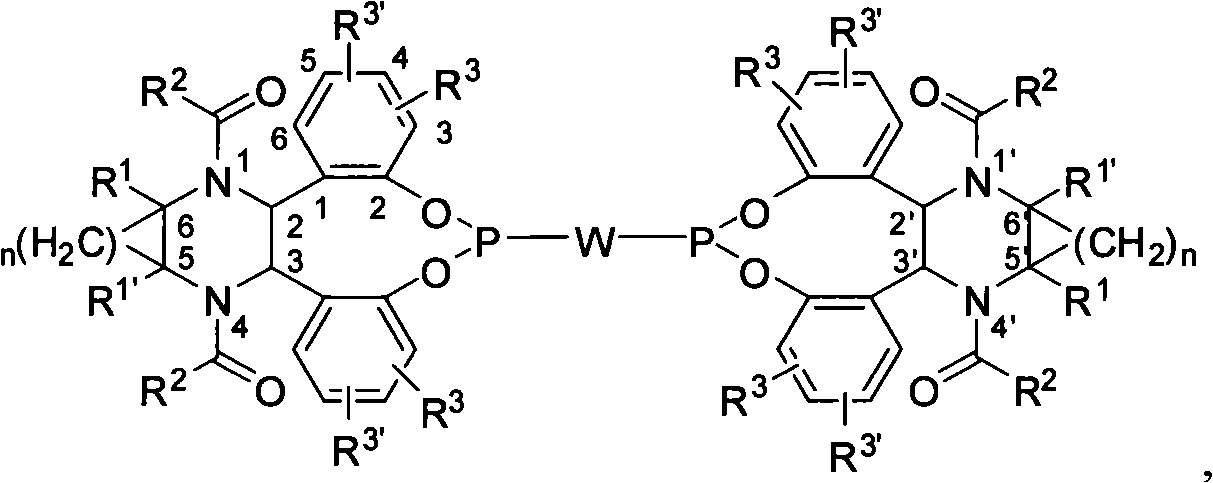
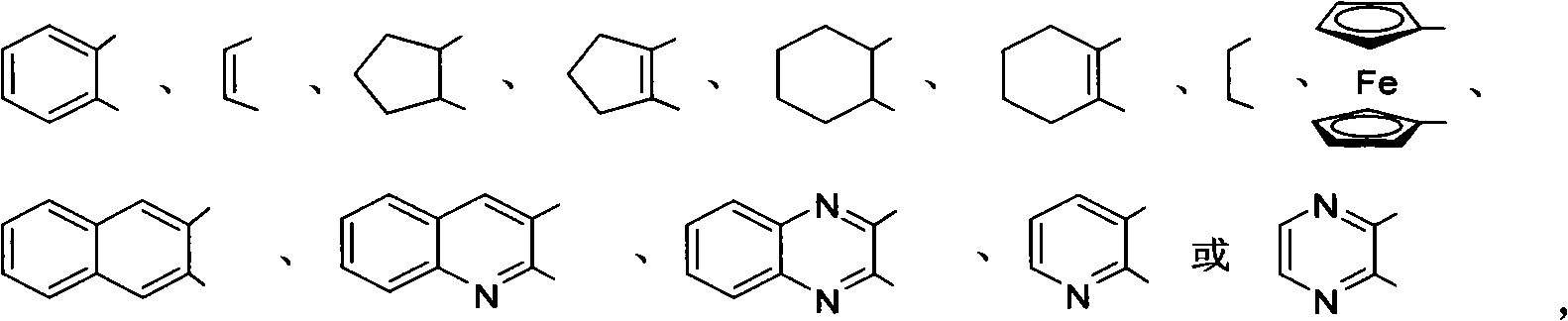
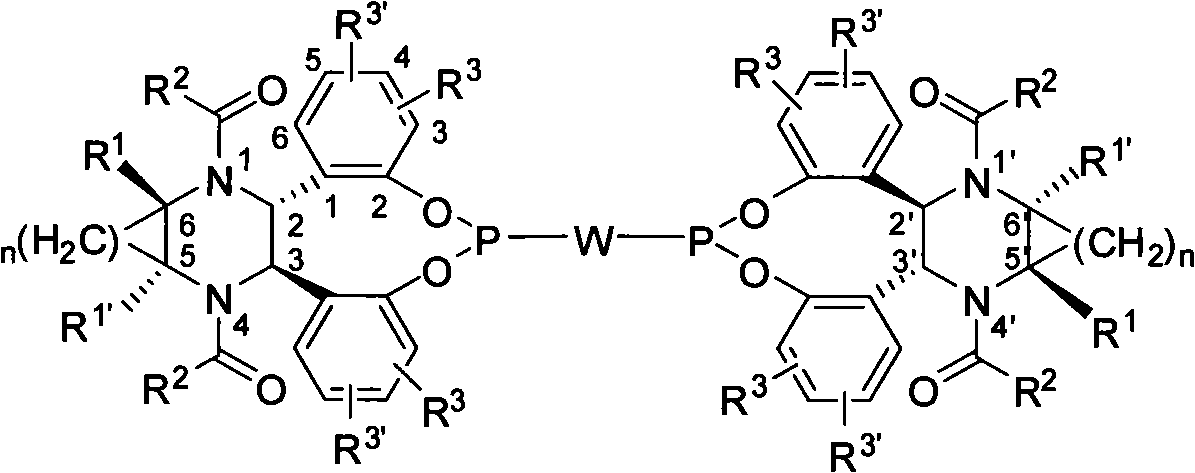
Bidentate phosphite ester ligand, synthetic method and use thereof in alkene unsymmetrical catalysis hydroformylation reaction
A bidentate phosphite and ligand technology, applied in organic compound/hydride/coordination complex catalysts, carbon monoxide reaction preparation, chemical instruments and methods, etc., can solve unsatisfactory product regioselectivity and harsh reaction conditions , low activity and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1: Compound 4a of (2R, 3R, 5S, 6S) configuration (wherein R 3 , R 3 '=H)
[0058] A 500ml there-necked flask, add (S, S) configuration 1,2-cyclohexanediamine (5.0g) and absolute ethanol (50ml) in the bottle, drop salicylaldehyde 2a (11.2 g) in ethanol (20mL), reflux for 12h after the dropwise addition. The reaction was stopped, cooled to room temperature, the solvent was evaporated under reduced pressure, and the oil was pumped dry to obtain an orange oil. The oil was dissolved in a mixed solvent of acetonitrile (180mL) and toluene (20mL), Mn powder (5.3g) was added to the solution under an argon atmosphere, the system was cooled to 0°C, and trifluoroacetic acid (15.7g) was added dropwise. mL), then stirred at room temperature for 24 hours. Stop the reaction, cool to 0°C, then add trifluoroacetic acid (7.9mL), precipitate a large amount of solid, filter, wash with petroleum ether (20mL×2) to obtain a white solid, dissolve the solid in 100mL water, saturated ...
Embodiment 2
[0060] Example 2: Preparation of (2S, 3S, 5R, 6R) configuration compound 4b (wherein R 3 = 3-Me,R 3 '=H)
[0061] Using the method of Example 1, the first step is to react cyclohexanediamine in (R, R) configuration with 3-methylsalicylaldehyde, and the yield is 78%.
[0062] Colorless needle-like solid; melting point 188-189°C; [α] 20 D -59(c 0.3, CHCl 3 ); 1 H NMR (300MHz, CDCl 3 )δ=1.39-1.42(m, 4H), 1.75-1.82(m, 4H), 2.23(s, 6H), 2.37(broad s, 2H), 2.68(broad, 2H), 4.13(s, 2H), 5.97(d, 2H, J=8.1Hz), 6.32(t, 2H, J=7.7Hz), 6.93(d, 2H, J=7.2Hz), 11.06(broad, 2H); 13 C NMR (75MHz, CDCl 3)δ=155.0, 130.0, 127.8, 125.1, 122.6, 118.0, 63.3, 59.7, 31.6, 24.3, 15.8; 925,745cm -1 ; EI-MS: m / z=352 (M + , 5.8), 254 (1.8), 217 (44), 136 (100); elemental analysis (%): theoretical value C 22 h 28 N 2 o 2 : C 74.97, H 8.01, N 7.95; Experimental value: C 74.73, H 8.13, N 7.82.
Embodiment 3
[0063] Example 3: Compound 4c of (2S, 3S, 5R, 6R) configuration (where R 3 = 4-Me, R 3 '=H)
[0064] Using the method of Example 1, the first step is to react cyclohexanediamine in (R, R) configuration with 4-methylsalicylaldehyde, yield: 78%.
[0065] Colorless needle-like solid; melting point 216-217°C; [α] 20 D -67(c 0.3, CHCl 3 ); 1 H NMR (300MHz, CDCl 3 )δ=1.38-1.41(m, 4H), 1.65-1.81(m, 4H), 2.21(s, 6H), 2.61(broad, 2H), 4.04(d, 2H, J=2.1Hz), 6.00(d , 2H, J=7.5Hz), 6.25(d, 2H, J=7.5Hz), 6.63(s, 2H), 10.79(broad, 2H); 13 C NMR (75MHz, CDCl 3 )δ=156.6, 138.8, 130.0, 120.3, 119.4, 117.1, 63.1, 59.7, 31.5, 24.3, 21.1; 1120,808cm -1 ; EI-MS: m / z=352 (M + , 8.8), 217 (51.9), 136 (100); elemental analysis (%) theoretical value C 22 h 28 N 2 o 2 : C 74.97, H 8.01, N 7.95; Experimental values: C 74.79, H 8.03, N 7.74.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com